2015 ATS Guidelines on Treatment of IPF Released
ATS Releases New Idiopathic Pulmonary Fibrosis Treatment Guidelines
Recent Studies Bring New Recommendations
This month, the American Thoracic Society, European Respiratory Society, Japanese Respiratory Society and Latin American Thoracic Association released their updated guidelines on the treatment of idiopathic pulmonary fibrosis (IPF). They are free on the ATS website for all to peruse, but here, we’ll give you the highlights of the data-rich 17-page-document.
These guidelines represent a much-needed update from the 2011 version as important new randomized controlled trials about IPF therapies have been published since then. It’s inspiring to note that the new guidelines are dedicated to the memory of Mr. William Cunningham (1935-2014), a patient who suffered from IPF and actively participated in the development of the guidelines.
The guidelines were framed as answering twelve important clinical questions in the treatment of IPF. Here’s a pictorial representation of how the new guidelines differ from the old ones:
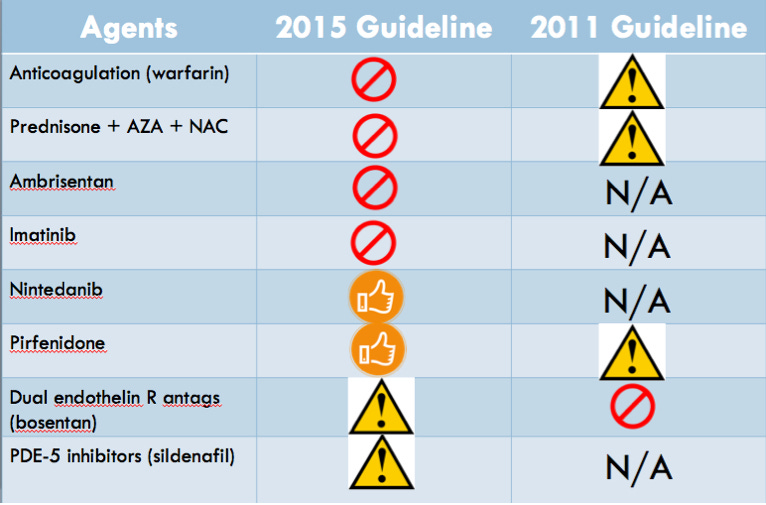
The X sign indicates that the committee gave a strong recommendation against use, the caution sign indicates a conditional or weak recommendation against, and the thumbs-up sign indicates a conditional or weak recommendation for use.
A Closer Look at the Recommendations
The strong recommendation against warfarin came largely from the 2012 Noth et al. RCT, which randomized 145 patients with IPF to warfarin versus placebo and was stopped early due to lack of benefit and significantly increased mortality with warfarin. The committee gave a strong recommendation against imatinib, the selective tyrosine kinase inhibitor, mostly due to an RCT showing no mortality benefit and increased adverse events in the imatinib group. However, nintedanib, a non-selective inhibitor of several tyrosine kinases got a conditional recommendation from the committee. The pooled analyses of the Richeldi et al article, and the INPULSIS-1 and INPULSIS-2 trials showed a RR of 0.70 for mortality and HR of 0.47 for acute exacerbations. Even though the individual trials didn’t show a mortality benefit, the committee felt that the “potential benefit on patient-important outcomes” made it worth recommending. Diarrhea was the major adverse event noted in the trials, and the committee recommended informing patients about this before committing to the treatment. The other hot new drug that got a conditional recommendation was pirfenidone, an anti-fibrotic medication whose mechanism still is not well-understood. Once again, the CAPACITY and ASCEND randomized double-blind placebo trials themselves did not individually show a mortality benefit, but the pooled results suggested improved mortality with a RR of 0.7. The main side effects to warn patients about include photosensitivity, fatigue, abdominal discomfort, and anorexia. The committee recommended further research into patients with emphysema as a comorbidity, a common patient population we see as clinicians. Ambrisentan, a selective ER-A endothelin receptor antagonist earned a strong recommendation against its use, based on the ARTEMIS-IPF RCT that was stopped early for lack of benefit, and the dual endothelin receptor antagonists bosentan and macitentan earned a weak recommendation against their use. The increased mortality and hospitalization rates noted in the PANTHER-IPF trial of combination prednisone + azathioprine + NAC earned a strong recommendation against this cocktail’s use. The decision about sildenafil, the PDE-5 inhibitor, was one of the more controversial amongst the committee. Although the committee gave a conditional recommendation against its use, due to the lack of mortality benefit or other respiratory outcomes (FVC, DLCO, 6-minute walk test distance, etc.), the secondary outcomes of quality of life measures were improved in the two main RCTs examined. Moreover, a subset of patients with right ventricular dysfunction may have seen some benefits, although more research needs to be done.
Some Recommendations Stay the Same
No changes were made from previous recommendations for the following therapies:
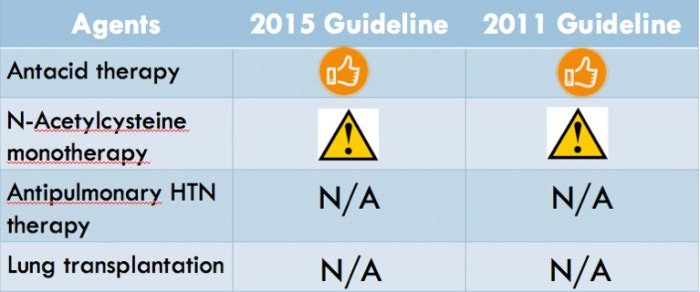
The relatively benign antacid therapies continued to get a conditional recommendation for their use. A Japanese RCT and an analysis of the NAC component of the aforementioned PANTHER-IPF trial re-affirmed the recommendation against using NAC as monotherapy. The committee punted decisions about single versus double-lung transplantation and antipulmonary hypertension therapy to the next iteration of guidelines.
Clinical Takeaway: The committee synthesized the latest and greatest in IPF evidence-based medicine since 2011 to come up with recommendations for and against the newer IPF drugs, while also taking a look at newer data published on our existing IPF therapies. Although no medications made the cut for a strong recommendation for use, both pirfenidone and nintedanib are promising contenders that earned the conditional recommendation. More than anything, the guidelines highlighted how there are still significant gaps in our understanding of the pathophysiology of IPF, and we’ll need even more research to honor the lives of Mr. Cunningham and others. Read More: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline


