Beta-blockers for septic shock (Review)
Blunting catecholamine toxicity without causing harm: is it possible?
Patients in septic shock are under extreme stress and have high levels of circulating catecholamines. Catecholamine toxicity has been postulated to worsen the organ failure associated with severe sepsis.
Vasopressor infusions with norepinephrine and epinephrine could theoretically add to catecholamine toxicity.
Infusions of ultra-short acting beta blockers such as esmolol and landiolol have been investigated as adjunctive therapies for patients with septic shock who have persistent tachycardia after treatment of pain, agitation, arrhythmias, etc.
We’ve reviewed these trials over the years, some of which have reported astounding reductions in mortality:
And a 2022 meta-analysis of 7 randomized trials seemed to verify the finding:
Then the STRESS-L trial (JAMA 2023) found greater mortality in the beta-blockade arm, although authors speculated this was due to a chance increase in withdrawal of care in the intervention patients.
A new meta-analysis by Sota et al (Chest 2025) brings together the eight randomized trials published since 2013, and made a crucial decision to stratify them by single- vs. multicenter designs.
Beta-Blockade for Sepsis: Single-Center Trials
The single-center trials together show a strong signal that ultra-short beta-blockade reduces mortality for patients with septic shock and persistent tachycardia.
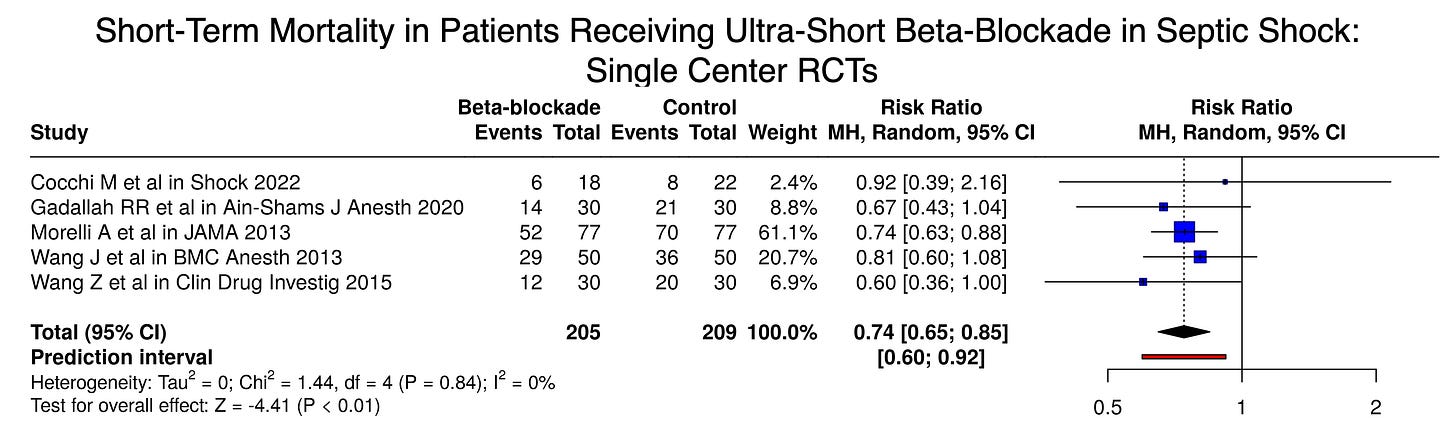
From these 414 patients enrolled in randomized trials at single centers in Egypt, China, Rome, and Boston, the answer seems clear: beta-blockers save lives in septic shock with a massive 26% relative risk reduction in mortality.
So why haven’t we all got on board with this innovative new therapy?
Beta-Blockade for Sepsis: Multicenter Trials
Ultra-short beta blocker infusions have been studied in small multicenter randomized trials, as well. Here, the effect seen in the single-center trials was not borne out. Just the opposite, in fact:
In the multicenter trials we see a trend toward increased mortality with beta-blockade for sepsis (RR 1.09).
Pooling all the trials together, the addition of Whitehouse et al (STRESS-L JAMA 2023) eliminates the statistical significant mortality benefit seen in the earlier meta-analysis, with a pooled risk of 0.84 (favoring beta-blockade, but with a 95% confidence interval of 0.68 to 1.02):
The heterogeneity of the included trials was significant, and authors’ trial sequence analysis and sensitivity analysis suggested fragility of the findings, further weakening confidence in the pooled risk ratio.
As one example, all the single-center trials tested esmolol, but all the multicenter trials tested landiolol.
One of the strongly positive trials (Morelli et al) used a protocol requiring pulmonary artery catheterization, measuring central venous oxygen saturations and administering the inotrope levosimendan to patients with low ScVO2. Other trials used noninvasive measurement of cardiac index and stroke volume, or none.
Conclusion
Besides theoretically blunting the deleterious effects of excess catecholamines, beta-blockers have negative inotropic and chronotropic effects. During septic shock, some patients’ tachycardia is likely adaptive, and suppressing it might be harmful.
After two of three multicenter trials produced results concerning for increased mortality with beta blockers, it’s hard to see adoption of this therapy in the near future.
Future studies should only be conducted under protocols that include hemodynamic monitoring of stroke volume. Two randomized trials are currently listed on clinicaltrials.gov testing landiolol, and several more testing esmolol, but none are designed to be adequately powered to provide an answer to this question.







Thank you for this!
Once again ... an outstanding review.