Endovascular therapy helps in ischemic stroke, again (ESCAPE)
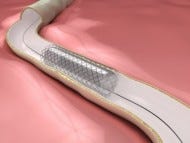
Endovascular Therapy Improves Outcomes from Ischemic Stroke
By Parth Rali, MD and Igor Titoff, DO
Endovascular therapy for ischemic stroke has long been an attractive treatment modality for ischemic strokes, but until recently large randomized trials have not confirmed a benefit [1,2,3]. Two of these—IMS III1 and SYNTHESIS2—failed to prove the benefit of endovascular therapy (with-or-without tissue plasminogen activator) over tPA alone, and the IMS III trial was stopped prematurely for futility [1]. Another recent trial, MR RESCUE3 (February 2013), also found no benefit of endovascular therapy over tPA. Thus tPA has remained the gold standard of intravascular therapy for acute ischemic stroke [4]. [For a "gold standard" therapy, tPA for treatment of ischemic stroke is surprisingly controversial. Some experts argue tPA should not be used for stroke outside clinical trials, and many emergency medicine physicians have declined to provide tPA, citing fear of lawsuits. --PulmCCM editors] Although these trials failed to show meaingful benefits from endovascular treatment, they did suggest important factors that could result in additional benefit with endovascular treatment:
proximal vessel occlusion;
a small infarct core (the amount of brain tissue around the infarct that is still potentially salvageable, a.k.a. penumbra);
specialized stroke teams who can deliver interventions quickly with high reperfusion rates.
The recently published ESCAPE trial in the New England Journal of Medicine used these factors as enrollment criteria. In this cherry-picked group of patients, endovascular therapy with tPA was markedly superior to tPA alone in treatment of ischemic stroke. ESCAPE follows the MR CLEAN trial, which also showed benefit of endovascular therapy added to tPA. ESCAPE investigators enrolled 315 patients with acute ischemic stroke at 22 centers worldwide, randomizing them to receive tPA alone, or tPA plus intra-arterial thrombectomy with devices like retrievable stents. Only patients with proximal intracranial arterial occlusion, small infarct core, moderate-to-good collateral circulation who were functionally independent before onset of stroke symptoms were enrolled. There was no age restriction. ESCAPE's results were remarkable: 53% of patients receiving endovascular therapy with tPA were functionally independent at 90 days, compared to 29% in those receiving tPA alone. Mortality was reduced by almost half: 10% of patients in the endovascular treatment arm died, compared to almost twice as many in the tPA only arm (19%). There were numerically more intracerebral hemorrhages in the endovascular group (3.6% vs 2.7%), but this was not statistically significant. ESCAPE was the rare example of a trial stopped early for efficacy, rather than futility: with a large observed benefit and the recently published MR CLEAN trial corroborating ESCAPE's results, the committee made the decision to terminate the trial. A discussion of the possible statistical implications of this decision and the trial design can be found elsewhere.
Endovascular Treatment for Stroke: A New Care Standard?
ESCAPE and MR CLEAN together create a convincing benefit of endovascular therapy added to tPA for highly selected patients. However, it will not be easy to duplicate these results by most centers. In fact, it will be impossible to consistently do so outside stroke centers of excellence, which for multiple reasons can today only exist in major urban areas. For that reason, ESCAPE and MR CLEAN don't speak to the individual physician -- emergency medicine, intensivist, neurologist, interventional radiologist, vascular surgeon or neurosurgeon. The proper audience for these trials are hospital administrators, physician leaders, and policymakers. The only way to reap the benefits ESCAPE and MR CLEAN suggest are possible is by organizing regional networks (hubs) around stroke centers of excellence that incorporate protocolized diagnostic methods, digital sharing of neuroimaging, and rapid transport of patients to the stroke center by ambulance or helicopter. This would be analagous to changes in the health system that occurred as reperfusion treatment for ST-elevation myocardial infarction evolved, albeit on a smaller scale. This shift is already occurring in major U.S. cities, and given the enormous burden of stroke on patients, families and society (and the advantages of stroke centers beyond these trials) the process should be encouraged by policymakers. In the U.S., one major obstacle to such arrangements is the presence of multiple competing centers in most metropolitan areas. This situation may be tolerable for an interim period, but after a certain observation/competition period, one center (or maybe 2 in large cities) should be awarded winner-takes-all.
Clinical Takeaway: Endovascular treatment added to tPA might dramatically improve outcomes for highly selected patients with ischemic strokes. Bringing these benefits to patients outside clinical trials will require system-level changes to rapidly identify and transport patients to major stroke centers. References: ESCAPE Trial: NEJM 2014 1 Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 2013;368:893–903 2. CicconeA, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischemic stroke N Engl J Med 2013;368:904 –13 3. Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke (MR RESCUE). N Engl J Med 2013;368:914-923 4. Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014 August 5 5. Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. (MR CLEAN) N Engl J Med 2015;372:11-20. [Erratum, N Engl J Med 2015;372:394.] MR CLEAN PulmCCM commentary


