The Great Lactate Debate Part 1: should we be counting protons or strong ions?
Jon-Emile S. Kenny MD [@heart_lung]
“…. She was here on earth to grasp the meaning of its wild enchantment and to call each thing by its right name …”
-Boris Pasternak
Background
Over the last half-decade, there has been a distinct shift in the approach to lactate elevation. The long-held belief that elevated serum lactate requires tissue or cellular hypoxia has fallen away. Indeed, in sepsis, tissues bathed in oxygen and with fully functional mitochondria can produce large amounts of lactate. Part of the confusion stems from the terms anaerobic and aerobic; anaerobic chemistry simply means that oxygen is not required to proceed, but this doesn’t necessitate oxygen’s absence. Glycolysis [the breakdown of glucose to pyruvic acid] is always anaerobic; anaerobic glycolysis is occurring within you right now – as you read this. Glycolysis can proceed without oxygen, it was occurring within prokaryotes long before the eukaryotic revolution, the oxygen-containing atmosphere and mitochrondrial respiration.
Yet when we consider the equations that generate pyruvic and lactic acid from glucose, something curious and paradoxical is apparent – an irregularity was laid bare, most-prominently, by Robergs and colleagues over a decade ago. Consider the following:

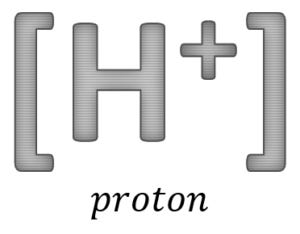
As can be seen, the breakdown of 1 glucose molecule results in 2 pyruvate molecules, 2 ATP, 2 NADH and 2 H+ [i.e. 2 protons]. Parenthetically, I will use the term ‘proton’ as a narrative device in this post; it will serve as a cognitive placeholder for the more complicated hydronium ion [H3O+]:H2On and other aqueous complexes [e.g. Eigen, Zundel] that form within the water matrix.
However, as seen below, the conversion of pyruvate to lactate, then consumes 2 protons, making the overall transformation of glucose to lactate a proton-neutral conversion.

If pH is the measure of proton activity, how can lactic acidosis [i.e. a fall in pH with a rise in lactate] occur?
The Great Debate
Thus, this post will briefly discuss the opposing positions with respect to the elaboration of the proton that is seen with hyperlactatemia. The first view, as mentioned in the Robergs paper and echoed in this relatively recent New England Journal Review is that the protons arise from biochemical work, that is:

So, in the ‘proton book-keeping’ paradigm, it is not really the lactate that is causing the acidosis, rather the creation of lactate is actually mitigating acidosis [by acting as the terminal electron and proton acceptor] when glycolytic flux is high. Accordingly, this represents a ‘biochemical-work induced’ acidosis with co-incident lactate elevation.
However, this position is challenged by a new [but not really] approach to acid-based known as the ‘strong ion approach.’ This paradigm was codified and made popular by the late Peter Stewart. It has a very different ontological and epistemological approach to acid-base than the Henderson-Hasselbalch perspective. Importantly, the Stewart approach maintains that protons [H+] themselves cannot independently alter the pH; the 3 independent variables [which the system alters] are:
the partial pressure of carbon dioxide [PaCO2]
the concentration of weak acids [Atot]
the strong ion difference [SID]
Crucially, these three variables form the boundaries within which the weak ion concentrations [e.g. [H+], [OH-]] dance towards their respective equilibrium constants.
As a consequence, the critical difference between these views is that – for the Stewartite – a change in pH can only be caused by a change in either PaCO2, Atot or SID; in other words, proton-counting is irrelevant as changes in proton concentration is the dependent variable. Instead, the physiologist should be counting the change in SID, Atot and or PaCO2 as these are the true determinants of pH.
Lactate Reduces the SID
As reviewed here previously, the strong ion difference is the ‘gap’ between all the fully dissociated cations [strong cations like sodium, magnesium, potassium, etc.] and fully dissociated anions [strong anions like chloride, sulfate, lactate]. The SID is normally positive, however, when strong anions like chloride or lactate accumulate, the SID falls in value. As this ‘positive’ gap collapses, weak cations like [H+] will rise in order to maintain electroneutrality. Thus, for the Stewartite, the addition of lactate – a strong anion – must increase the [H+] [i.e. lower the pH] because the SID falls and charge-balance must be kept.
Certainly, at least one response to the Robergs paper employed the Stewart approach to rebut the discrepancy between the observed acidosis and proton-book-keeping approach. The authors of this response describe the effects of changes in intracellular strong cations [like sodium and potassium], phosphocreatine [which is a strong anion, until it is cleaved] and other strong ions. Importantly, inorganic phosphate [Pi] is labeled a weak acid in their response; however, like albumin, placing inorganic phosphate distinctly in the category of Atot is not so clear. As mentioned in part 2, phosphate has three pKas, the lowest being nearly 2.0 [ironically, lower than lactate]. Accordingly, inorganic phosphate always carries at least 1 negative charge and acts – partially – as a strong anion.
And as I read this response – like other proponents of the SID – I couldn’t help but wonder the strong ion status of the following: NAD+, FADH+, Mg-ATP2-, Mg-ADP1-, uric acid, 3-phosphoglycerate, 2-phosphoglycerate, the various phosphorylated protein intermediates, amino acids etc. etc. Knowing the instantaneous SID for all species within the cell at any time demands very high-computing power – bordering upon omniscient computing power. Indeed, the initial foundations of the Stewart paradigm required solving a fourth-order polynomial equation made convenient in 1980 because of microprocessors. But to consider all of the chemical entities floating around the cell – converting their strong charges to weak charges, or to weak acids and back – I was reminded of Matthew 10:29
“But not a single sparrow can fall to the ground without your Father knowing it.”
So, instead of simplifying our approach to acid-base, the Stewart approach seems to complicate it – demanding God-like computational prowess. Despite this criticism, however, we must accept objective truth as the arbiter of validity, not complexity.
I turn to Carl Sagan:
“Extraordinary claims require extraordinary evidence.”
The Stewart approach is an extraordinary claim; does the evidence follow? More on that in Part 2
Best,
Dr. Kenny is the cofounder and Chief Medical Officer of Flosonics Medical; he is also the creator and author of a free hemodynamic curriculum at heart-lung.org


