ICU Physiology in 1000 Words: Weaning-Induced Cardiac Dysfunction & the Passive Leg Raise
Jon-Emile S. Kenny MD [@heart_lung]
Reminder: Help me with my master’s thesis! Please complete a learning module, and fill out this exceptionally brief survey!
Perhaps the landmark trial elaborating an evolving cardiac dysfunction during the spontaneous breathing trial [SBT] is that of Lemaire and colleagues – published in 1988 [1]. One particularly memorable patient of mine who miserably failed extubation despite a normal Tobin Index led me to Lemaire’s classic investigation and prompted this summary of the dizzying physiology at play as a patient switches from mechanically-assisted to unassisted breathing. Notably, the second author on Lemaire’s landmark study – Jean-Louis Teboul – has published an elegant review of their initial work [2] which should be mandatory reading for all house-officers.
Left Atrial Pressure Rising
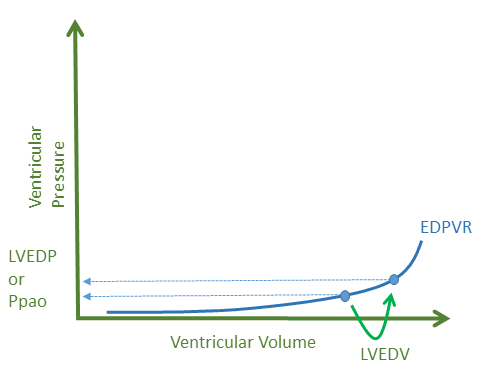
A maelstrom of mechanisms ceaselessly spiral towards an increased left ventricular [LV] filling pressure when a patient is transitioned from assisted ventilation to a T-piece SBT [3]. In patients with preserved LV diastolic filling properties, an increase in left ventricular end-diastolic volume [LVEDV] will raise the left-ventricular end-diastolic pressure [LVEDP] commonly obtained from a pulmonary artery catheter as the pulmonary artery occlusion pressure [Ppao] - see figure 1.
Figure 1: EDPVR is end-diastolic pressure volume relationship, LVEDV is left ventricular end diastolic volume increasing on the x-axis with rise of left ventricular end diastolic pressure [LVEDP] or pulmonary artery occlusion pressure [Ppao] on y-axis.While it is tempting to ascribe an increase in LVEDV to simply an increase in preload from the right ventricle [RV] as positive pressure is removed, diminished cardiac contractility and increased LV afterload will also raise LVEDV and therefore the LV filling pressure - see figure 2.
Figure 2: EDPVR is end-diastolic pressure volume relationship, ESPVR is end-systolic pressure volume relationship. The blue loop represents normal state with LVEDV at point 1. The red loop is an increase in afterload with LVEDV at point 2. The green loop represents diminished LV contractility with LVEDV at point 3.
Each of these mechanisms are completely plausible as breathing work and adrenergic tone rise during the SBT exercise [2]. In patients with pre-existing coronary artery disease, flow resistance will render the heart susceptible to ischemia especially as the demands of respiratory muscles intensify [4]; impaired cardiac contractility seems particularly troubling in patients with an underlying impaired ejection fraction [5]. Yet, rising LV afterload will also retard egress of blood from the LV and raise its filling pressure. Increased blood pressure during the SBT should raise concern for this mechanism, but the absence of elevated blood pressure certainly does not rule out a heightening of LV outflow impedance [i.e. afterload] [6]. Deep negative inspiratory efforts during systole will raise the trans-mural LV and aortic pressure thereby afterloading the LV and raising its LVEDV and LVEDP [7].
Equally-insulting to the LV, and probably occurring much more commonly, is the presence of diastolic incompetence. Diastolic dysfunction of the LV was noted even in the initial Lemaire investigation as a subset of patients demonstrated little-or-no increase in LVEDV, but a marked rise in transmural LVEDP. What this indicates is a steepening of the left ventricular end-diastolic pressure-volume relationship - see figure 3.
Figure 3: EDPVR is end diastolic pressure volume relationship. The darkened EDPVR represents diastolic dysfunction, or a stiffened LV which may result from RV enlargement. Note that for the same LVEDV on the x-axis, the LVEDP is increased on y-axis.
Importantly, Moschietto and colleagues noted that patients who failed SBT were unable to increase their mitral annular tissue doppler velocity [Ea] which is a non-invasive echocardiographic index of LV relaxation [8]. By contrast, those who passed their SBT were able to increase LV relaxation. Similar physiology has been noticed in patients with exercise-induced diastolic dysfunction [9]. Crucially, in Moschietto’s study, early LV filling velocity increased in both those who passed and failed the SBT – the key difference being that early LV filling increased in those who passed the SBT because of their ability to increase LV relaxation. Consequently, the rise in early diastolic filling in those who failed the SBT was due not to more rapid relaxation, but instead, increased left atrial pressure.
The underlying etiology of evolving diastolic dysfunction during SBT may be that of pre-existing LV stiffness, dynamic LV ischemia and, interestingly, an encroaching interventricular septum secondary to an enlarged right ventricle [10], as described below.
The Engorged Right Ventricle
While it is commonly taught that removal of positive pressure increases the gradient for venous return, this isn’t entirely true [11]. Assisted breathing with positive end-expiratory pressure [PEEP], doesn’t diminish the gradient for venous return, rather, it increases the Pcrit which is the critical collapsing pressure of the great vessels as they enter the thorax. When this is removed, maximal venous return during spontaneous inspiration is increased - see figure 4.
Figure 4: the removal of positive pressure with PEEP results in an increase in maximal venous return and a fall in the critical collapse pressure of the great veins. Pra is right atrial pressure. Note that with removal of PEEP, Pcrit falls from ~ 8 mmHg to zero mmHg, or atmospheric pressure.
As venous return increases and more negative inspiratory pressures take hold, the right ventricular [RV] blood volume rises. As above, this may encroach upon the interventricular septum and stiffen the LV during diastole [10]. Within a few cardiac cycles, this increase in RV preload is transmitted to the LV and thus LVEDV and LVEDP will rise, placing the patient at risk for weaning-induced pulmonary edema [12].
While an enlarged RV will result from high inflow, poor outflow will contribute to its size. To the extent the hypoxemia and hypercapnia progress, the RV will afterload [2]. Additionally, rapid breathing in patients with expiratory airflow limitation [e.g. airway edema, ARDS, COPD, obesity] can lead to gas-trapping [13-15] which will raise RV outflow impedance [16]. If the lower-lobe lung volume rises, the mediastinum can be compressed which couples RV and LV filling – accentuating the effects of ventricular interdependence [17].
Predicting Cardiac Dysfunction
Given the above, predicting ‘weaning-induced pulmonary edema’ [WIPE] is important. Towards this end, a recent paper described the incidence of WIPE and clinical associations [18]. Importantly, amongst 81 patients undergoing an SBT via T-piece, 29 had at least 1 episode of WIPE – all of whom failed SBT; logistic regression revealed that the odds ratio of WIPE was greatest for those with known COPD [OR 8.7], followed by known ‘cardiopathy’ [defined as dilated, hypertrophic, hypokinetic or with significant valvular disease – OR 4.5] and also obesity [OR 3.6]. The use of the passive leg raise [PLR] to predict preload independence as a risk for WIPE [19] had been previously investigated by this group, thus it was used to predict WIPE again. In those where a PLR revealed preload independence [i.e. no augmentation of cardiac in response to a PLR], diuretics were given. 9 remained preload independent and of those, 7 had WIPE again. Of the 7 whose PLR revealed conversion to preload-dependence following diuretics, 6 went on to pass a subsequent SBT.
Importantly, preload independence does not necessarily reveal a patient’s volume status – volume status and volume responsiveness may diverge. Preload independence, does alert the clinician to a patient whose venous return curve [at any volume status] is intersecting the plateau of the cardiac function curve; such a patient has little cardiac reserve and is, accordingly, at risk for WIPE.
In addition to the use of the PLR to predict WIPE, data exists for BNP elevation as well as hemo-concentration [12]. While beyond the scope of this brief review, the diagnosis of WIPE is important as treatment with diuretics and/or afterload reduction is required to facilitate liberation from mechanical ventilation.
Please check out other posts in this series.
Best,
Lemaire, F., et al., Acute left ventricular dysfunction during unsuccessful weaning from mechanical ventilation. Anesthesiology, 1988. 69(2): p. 171-9.
Teboul, J.-L., Weaning-induced cardiac dysfunction: where are we today? Intensive Care Med, 2014. 40(8): p. 1069-1079.
Tobin, M.J., Extubation and the myth of "minimal ventilator settings". Am J Respir Crit Care Med, 2012. 185(4): p. 349-50.
Aaron, E.A., et al., Oxygen cost of exercise hyperpnea: measurement. J Appl Physiol (1985), 1992. 72(5): p. 1810-7.
Caille, V., et al., Echocardiography: a help in the weaning process. Critical care, 2010. 14(3): p. R120.
Richard, C., et al., Left ventricular function during weaning of patients with chronic obstructive pulmonary disease. Intensive Care Med, 1994. 20(3): p. 181-186.
Buda, A.J., et al., Effect of intrathoracic pressure on left ventricular performance. N Engl J Med, 1979. 301(9): p. 453-9.
Moschietto, S., et al., Transthoracic echocardiography with Doppler tissue imaging predicts weaning failure from mechanical ventilation: evolution of the left ventricle relaxation rate during a spontaneous breathing trial is the key factor in weaning outcome. Critical care, 2012. 16(3): p. R81.
Nonogi, H., et al., Diastolic properties of the normal left ventricle during supine exercise. British heart journal, 1988. 60(1): p. 30-38.
Kasner, M., et al., Left ventricular dysfunction induced by nonsevere idiopathic pulmonary arterial hypertension: a pressure-volume relationship study. Am J Respir Crit Care Med, 2012. 186(2): p. 181-9.
Fessler, H.E., Heart-lung interactions: applications in the critically ill. Eur Respir J, 1997. 10(1): p. 226-37.
Dres, M., J.-L. Teboul, and X. Monnet, Weaning the cardiac patient from mechanical ventilation. Curr Opin Crit Care, 2014. 20(5): p. 493-498.
Vieillard-Baron, A., et al., Pressure-volume curves in acute respiratory distress syndrome: clinical demonstration of the influence of expiratory flow limitation on the initial slope. Am J Respir Crit Care Med, 2002. 165(8): p. 1107-12.
Ishii, M., et al., Effects of hemodynamic edema formation on peripheral vs. central airway mechanics. J Appl Physiol (1985), 1985. 59(5): p. 1578-84.
Salome, C.M., G.G. King, and N. Berend, Physiology of obesity and effects on lung function. J Appl Physiol (1985), 2010. 108(1): p. 206-11.
Harris, P., et al., The influence of the airways resistance and alveolar pressure on the pulmonary vascular resistance in chronic bronhcitis. Cardiovasc Res, 1968. 2(1): p. 84-92.
Butler, J., et al., Cause of the raised wedge pressure on exercise in chronic obstructive pulmonary disease. Am Rev Respir Dis, 1988. 138(2): p. 350-4.
Liu, J., et al., Cardiac dysfunction induced by weaning from mechanical ventilation: incidence, risk factors, and effects of fluid removal. Critical care, 2016. 20(1): p. 369.
Dres, M., et al., Passive leg raising performed before a spontaneous breathing trial predicts weaning-induced cardiac dysfunction. Intensive Care Med, 2015. 41(3): p. 487-494.