Mechanical Circulatory Support Devices: What You Need to Know (Part 1 of 2)
The Rise of Mechanical Circulatory Support Devices
What Critical Care Physicians Need to Know
Part 1 of 2 (read part 2)
I. The failing pump and hemodynamic rationale for the use of MCS devices The rising field of mechanical circulatory support (MCS) offers a spectrum of therapies and devices with the potential to rescue patients with life-threatening cardiogenic shock1. Traditionally within the domain of cardiothoracic surgeons and interventional cardiologists, the expansion of indications for some of these support strategies outside the operating room and cardiac catheterization lab creates the need for multidisciplinary teams to care for these patients. Emergency physicians and intensivists must be familiar with developments in this field, as they often are the first providers to encounter the patient and therefore play a key role in directing their care during the crucial initial hours. Understanding the vicious cycle of pump failure Cardiogenic shock is often defined as sustained hypotension (SBP < 90 mmHg or 30 mmHg bellow known baseline), low cardiac output (CO) defined by cardiac index (CI) < 2.2 l/min/m2, high central filling pressures defined by pulmonary capillary wedge pressure (PCWP) > 12 mmHg and signs of diminished tissue perfusion. From a physiological standpoint, what specifically distinguishes cardiogenic shock is the mechanical impairment of the pump function (contractility), which leads to insufficient CO. When patients develop pump failure, they enter a vicious cycle of intra-cardiac and systemic changes, which lead to a further decrease in the SV. Increased LV end-diastolic pressures lead to increased LV wall tension, which leads to coronary ischemia affecting contractility. The systemic hypotension resulting from the fall in SV leads to tissue hypoperfusion with subsequent acidemia, which further impairs contractility.
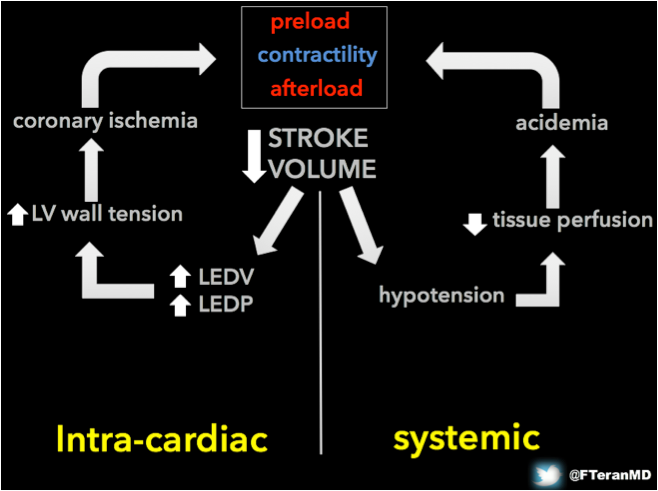
The role and hemodynamic effects of MCS The treatment of cardiogenic shock is directed to break this cycle and is two-fold: fix the triggering factor and support pump function. If the underlying problem is evident, such as an acute myocardial infarction, an acute valvular failure or a medication overdose (e.g. calcium channel blocker overdose), specific therapies should be instituted. However, in many cases like the one described here, the heart will need time (hours, days or weeks) to recover. In these cases, the mainstay therapy is “buying time” for the patient. Inotropic agents are the first line therapy in cardiogenic shock and they attempt to break the cycle by directly augmenting contractility (beta-adrenergic effect or phosphodiesterase-3 inhibition) as well as improving systemic hypoperfusion by increasing vascular resistance (alpha effect). While these agents (e.g. epinephrine, dobutamine, milrinone, etc.) all have the potential to improve the hemodynamics and stabilize some patients, when there is severe failure, pharmacologic therapy alone is generally going to come at very high price from the perspective of both the myocardium and the peripheral and splanchnic circulations. Beta-adrenergic stimulation may improve contractility of areas that are perfused, but it will greatly increase myocardial oxygen demand, feeding into and fueling the vicious cycle. Alfa-adrenergic vasoconstriction may improve coronary and systemic perfusion pressures, but will increase both systemic and pulmonary vascular resistance2, making it harder for failing ventricles to maintain ejection fraction. It will also leave the peripheral and splanchnic beds vasoconstricted and tissues underperfused. This is where MCS comes into play. Mechanical circulatory support involves a wide range of devices that share the common function of supporting or even replacing the pump function of the failing ventricle. They decrease LV wall tension, improving coronary flow and contractility. Unlike inotropic agents, MCS devices restore the balance between myocardial oxygen supply and demand, and generate effective systemic perfusion. Depending on the location of the pump, MCS devices are classified as intracorporeal (an implanted device), percutaneous or extracorporeal. They are also classified as short term (or rescue), and long-term therapies, according to the time they are intended to provide support for. Examples of intracorporeal devices include left, right or biventricular Assist Devices (VADs) and the Total Artificial Heart. Percutaneous devices include the intra-aortic balloon pump (IABP), the Impella®, and the TandemHeart®. Extracorporeal devices include the Centrimag®, Rotaflow® and the CardioHelp®. Classification of devices, mechanisms, hemodynamic effects and common indications are summarized in Table 1 in part 2 of this post. Why is it important that acute care physicians are familiar with the field of MCS? Given the rapidly growing number of patients with advanced heart failure who are being managed with long-term MCS devices (specifically LVADs), emergency physicians and other acute care providers are encountering these patients with increasing frequency3. Patients with implanted devices may present to the ED with acute complaints and MCS–related complications that clinicians must understand to appropriately manage4. For instance ventricular arrhythmias, driveline infections, GI bleed or suction events in the LVAD patient5. Furthermore, the large experience gained in the field of MCS in long-term support strategies over the past decade, along with significant improvements in the technologies (making more durable and safer devices, with easier implantation), has led to the expansion to short-term applications. This experience has also prompted the development of new devices specifically designed to provide emergency support (e.g. Impella® or CardioHelp® ECMO). These applications are still largely supported only by observational data but if patients have been successfully supported with these life-saving therapies when emergencies have occurred in the operating room6 or the cardiac catheterization lab7, it seems reasonable to offer the same opportunity to selected patients in the ED or ICU. Patients in the ED who could potentially benefit from MCS, will never get access to them unless the emergency provider considers this possibility and contacts the appropriate teams early enough to avoid perpetuating the vicious cycle of pump failure to a point of irreversible organ damage. As stated in the 2015 SCA/ACC/HFSA/STS consensus recommendations on the use of percutaneous MCS devices: “Early initiation of MCS support can mitigate the consequences of systemic hypoperfusion, worsening acidemia, and declining cardiac function”8. Indications for Mechanic Circulatory Support Classic long-term indications for MCS include bridge to transplant and destination therapy9. In bridge to transplant a patient with advanced non-reversible heart failure with an indication for cardiac transplant can be bridged with MCS until the organ is available and / or the patient is fit for transplant. In destination therapy MCS devices are implanted as a life-long strategy for patients who are not candidates for transplantation. Emerging short-term indications include bridge to recovery and immediate survival. Bridge to recovery: this is the case of the patient described above. A patient with acute severe heart failure, with a known recoverable underlying etiology can be transiently supported with a mechanical device until native function is recovered. Bridge to immediate survival: this is a new and promising indication that includes the deployment of either percutaneous or extracorporeal devices during a life-threatening event. In this group we have any confirmed or suspected treatable precipitating etiology. For instance, a patient with an acute MI who repeatedly goes into VF and needs to be bridged to have PCI, or a patient with massive PE who is bridged to go for thrombectomy10.
Felipe Teran-Merino M.D.
Divisions of Emergency Ultrasound and Emergency Critical Care
Department of Emergency Medicine
Icahn School of Medicine at Mount Sinai
A version of this post was originally published in the newsletter of the American College of Emergency Physicians. References:
Eckman PM, Hryniewicz K. Prime Time for Temporary Mechanical Circulatory Support. J Cardiovasc Transl Res. 2015 Jul;8(5):281-2.
Barnard MJ, Linter SP. Acute circulatory support. BMJ. 1993 Jul 3;307(6895):35-41.
Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015 Dec;34(12):1495-504.
Sen A, Larson JS, Kashani KB, Libricz SL, Patel BM, Guru PK, Alwardt CM, Pajaro O, Farmer JC. Mechanical circulatory assist devices: a primer for critical care and emergency physicians. Crit Care. 2016 Jun 25;20(1):153.
Burke MA, Givertz MM. Assessment and management of heart failure after left ventricular assist device implantation. Circulation. 2014 Mar 11;129(10):1161-6.
Desai N, Chaudhry K, Aji J. Impella left ventricular assist device in cardiac arrest after spinal anaesthesia for caesarean section. BMJ Case Rep. 2015 Oct 28;2015.
Heidlebaugh M, Kurz MC, Turkelson CL, Sawyer KN. Full neurologic recovery and return of spontaneous circulation following prolonged cardiac arrest facilitated by percutaneous left ventricular assist device. Ther Hypothermia Temp Manag. 2014 Dec;4(4):168-72.
Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, Kern M, Garratt KN, Goldstein JA, Dimas V, Tu T; 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care. J Am Coll Cardiol. 2015 May 19;65(19):e7-e26.
Shreenivas SS, Rame JE, Jessup M. Mechanical Circulatory Support as a Bridge to Transplant or for Destination Therapy. Current Heart Failure Reports. 2010;7(4):159-166. doi:10.1007/s11897-010-0026-4.
Spangenberg T, Meincke F, Brooks S, Frerker C, Kreidel F, Thielsen T, Schmidt T, Kuck KH, Ghanem A. "Shock and Go?" extracorporeal cardio-pulmonary resuscitation in the golden-hour of ROSC. Catheter Cardiovasc Interv. 2016 Jun 17.


