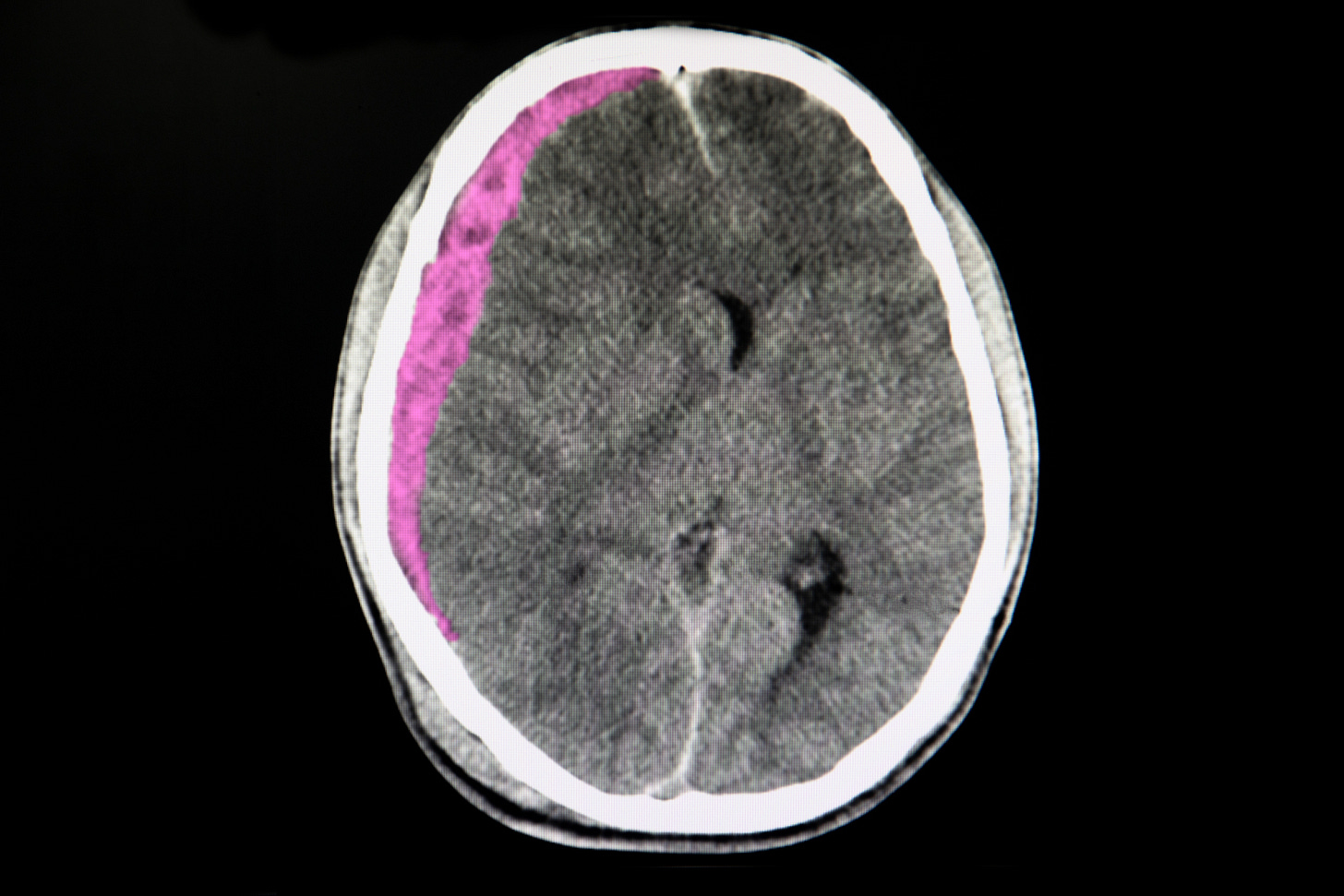
Middle meningeal artery embolization for subdural hematoma
EMBOLISE and MAGIC-MT trials test Medtronic's Onyx™ product
Adjunctive embolization in subacute or chronic subdural hematoma (EMBOLISE trial)
Middle meningeal artery (MMA) embolization can reduce the blood supply to dural vascular membranes, theoretically reducing the rate of subdural hematoma expansion or recurrence. Among 400 patients, those randomized to surgical evacuation plus MMA embolization with Medtronic’s Onyx™ liquid embolic product had a lower rate of hematoma recurrence or progression leading to repeat surgery (4%), compared to 11% for those randomized to surgery alone.
However, embolized patients had nominally higher rates of death at 90 days (5% vs ~3%), functional deterioration (~12% vs ~10%), and disabling stroke (2 patients), leading authors to suggest caution while awaiting further safety data on the procedure. Medtronic funded the trial; the lead author reportedly received $119,033 in direct payments from Medtronic (i.e., that were not associated with any research study) between 2017 and 2023, according to CMS.
Source: NEJM
MMA embolization instead of surgery in subacute or chronic subdural hematoma (MAGIC-MT trial)*
*in China
After surgeons had made the decision whether or not to operate on 772 patients with subacute or chronic subdural hematoma, subjects were then randomized to receive middle meningeal artery (MMA) embolization with Medtronic’s Onyx™ product, or usual care. Everyone involved was unblinded and bias was inevitably introduced at the time of the surgeon’s decision. That being said, there was no significant reduction in the recurrence or progression of subdural hematoma within 90 days (6.7% in the embolization group vs 9.9% in the usual-care group), but fewer serious adverse events occurred in the embolization group (6.7% vs. 11.6%, P=0.02).
Source: NEJM