Guidelines on Red Cell Transfusion (Review)
Recommendations are conservative, but most clinicians are still liberal
Anemia is the norm among critically ill patients, who were historically transfused to normal or near-normal hemoglobin levels in the hope of optimizing their physiology and chances for recovery.
In 1999, the Transfusion Requirements in Critical Care (TRICC) trial demonstrated that in a mix of patients with critical illness, restricting red blood cell transfusion to those with hemoglobin <7 g/dL did not worsen outcomes.
Patients with myocardial ischemia appeared to be an exception, and the subsequent MINT trial seemed to confirm the potential harms of a restrictive transfusion strategy in patients with MIs.
Since TRICC’s publication, dozens of randomized trials have been performed, together reinforcing (or at least not refuting) the safety of a restrictive transfusion strategy.
As the word got out, clinicians around the world noted the results, then kept transfusing patients as they pleased. Among 3,600 patients in 233 ICUs globally between 2019 and 2022, over 80% of transfusions were for patients with hemoglobin >7 g/dL.
Since 2020, the Association for the Advancement of Blood and Biotherapies (formerly called the American Association of Blood Banks) and major U.S. and European critical care societies have separately issued guidelines on red cell transfusion in hospitalized or critically ill patients.
We review the evidence supporting those guidelines here. PulmCCM has no affiliation with any specialty society.
General Critically Ill Patients (Not Bleeding, Hemodynamically Stable, Without MIs)
All societies on record have recommended a restrictive approach to red cell transfusion in hemodynamically stable patients without active bleeding or myocardial ischemia. These are generally classified as strong recommendations on moderately certain evidence.
AABB and the European Society of Intensive Care Medicine (ESICM) advised a transfusion trigger of hemoglobin less than 7 g/dL.
In its evidence review, a U.S. society found no adverse effects of restrictive transfusion strategies (Hb 7.0 to 8.0 g/dL) among more than 16,000 patients in multiple randomized trials.
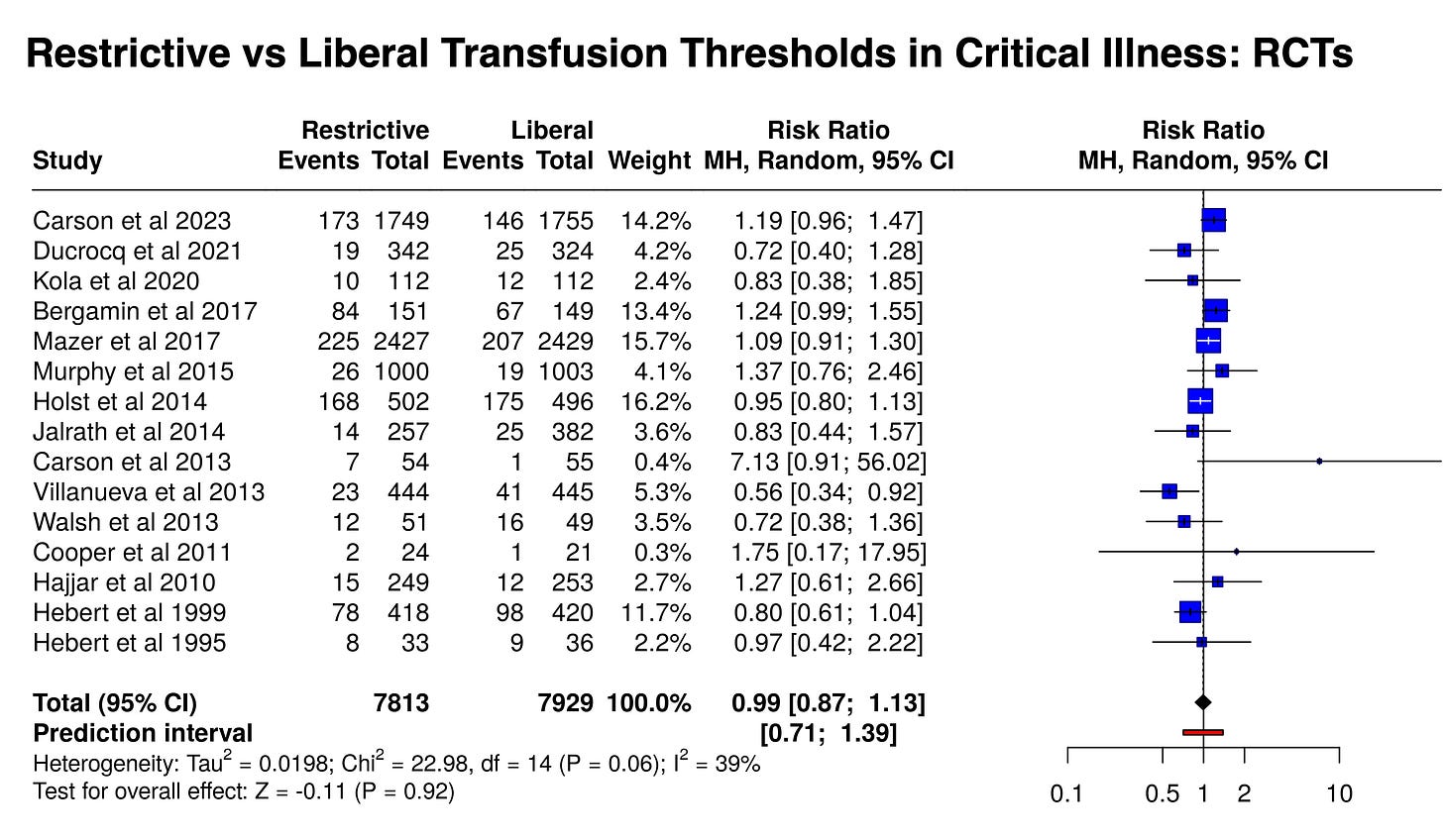
At least 15 trials enrolled patients with a diversity of critical illness, measuring short-term mortality as an outcome (28-45 days, mostly using 30 days). Here they are in a meta-analysis:
Active GI Bleeding
Keep reading with a 7-day free trial
Subscribe to PulmCCM to keep reading this post and get 7 days of free access to the full post archives.



