ICU Physiology in 1000 Words: The Mean Systemic Filling Pressure – Part 2
Jon-Emile S. Kenny MD [@heart_lung]
Briefly, part 1 of this reflection on the mean systemic filling pressure [Pmsf] considered an analogy for volume status as the vastness of an ocean beyond the hull of a leaking ship; I argue that looking only inside the hull of the ship cannot tell you the volume of the ocean – just as an echocardiographic volume assessment of the IVC or ventricle cannot establish the blood volume of a patient. Thus, an objective measure of volume status is described – the mean systemic filling pressure. Yet, like any pressure surrogate, relation to volume is complicated by the properties of the container in which the volume resides [i.e. the compliance of the chamber].
Measuring the Mean Systemic Filling Pressure
As described in part 1, the experimental method by which the Pmsf is determined is cessation of cardiac activity – arterial, capillary, venous and cardiac pressures equilibrate to the Pmsf. This settling pressure is determined by the stressed blood volume and vascular compliance. However, there are 3 ways by which the Pmsf has been approximated in vivo, without cessation of cardiac activity. The first exploits mechanical heart-lung interaction by simultaneously measuring right atrial pressure and cardiac output in response to a series of increasing inspiratory holds. Progressive escalation in intra-thoracic pressure raises right atrial pressure and diminishes venous return/cardiac output. If these values are plotted, a linear venous return curve is generated and Pmsf can be extrapolated as the x-intercept – see figure 1.
Figure 1: Instantaneous measurement of both central venous pressure [or right atrial pressure] and cardiac output is plotted at increasingly high inspiratory holds in a ventilated patient [e.g. 5, 15, 25, 35 cm H2O]. The extrapolated venous return curve can estimate the Pmsf in red. Central venous pressure is the x-axis and cardiac output is the y-axis.
Pinsky proposed this analysis decades ago in a dog model and confirmed the method of extrapolation to Pmsf measured by cardiac standstill [1]. Maas and colleagues have utilized inspiratory holds and extrapolation of the venous return curve as a method of determining Pmsf and found the average Pmsf in euvolemic post-cardiac surgery patients to be closer to 20 mmHg [2].
A second method of measuring Pmsf is to use the arm as a surrogate for the entire circulation. If a radial arterial line is in place, very rapid insufflation of a blood pressure cuff results in the venous and arterial pressures of the arm to fall towards the Pmsf; indeed, this has also been studied by Pinsky’s group [3] and found to correlate with the method of extrapolation described above.
Lastly, a mathematical approximation of the Pmsf has been proposed by Parkin and described in a superb review – an imperative read [4]. The Pmsf can be derived from knowing the right atrial pressure, cardiac output and some basic anthropometric variables. In the study which evaluated radial arterial pressure as a surrogate for Pmsf, Parkin’s formula was also studied. While there was considerable variability, the actual value of Pmsf by all three methods were close in value [i.e. 15 – 20 mmHg] [3].
Interestingly, all of the methods above yielded unexpectedly higher values of Pmsf [i.e. closer to 20 mmHg rather than 10 mmHg]. As noted by Repesse and colleagues, methods that utilize mechanical heart-lung interactions to extrapolate Pmsf assume that the venous return curve is linear [i.e. mechanical ventilation does not change the resistance to venous return] and that Pmsf itself will not change in response to an inspiratory hold. Importantly, mechanical ventilation is well-known to increase the right atrial pressure and the Pmsf – that is, the gradient for venous return does not vary [5-7]. The mechanism by which mechanical inspiration increases Pmsf is probably in response to sympathetic tone and/or redistribution of venous blood volume [8, 9].
Therapeutic Control of the Mean Systemic Filling Pressure
If Pmsf can be assessed, one must consider whether its manipulation has any clinical import. As in part 1, direct relationship between a patient’s blood volume and the Pmsf is tenuous because of the dynamic nature of the stressed volume in response to adrenergic tone; a low Pmsf may indicate hypovolemia, but also venoplegia or some combination thereof. Accordingly, clinical context is imperative. Another potential implication of Pmsf measurement is knowing venous tone or compliance. Measuring Pmsf following injection of a known volume of crystalloid allows measurement of venous compliance and potentially whether therapy should focus on adrenergic agents [i.e. to correct high venous compliance] or not [10]. Yet, provision of norepinephrine has multiple, conflicting effects on cardiac output including: augmenting Pmsf, increasing the resistance to venous return and down-shifting the cardiac function curve if afterload is increased by alpha stimulation [11].
Administration of volume has been shown to increase Pmsf [11]; interestingly, however, in fluid responsive patients, the gradient between the Pmsf and central venous pressure rose, whilst in fluid non-responsive patients, the gradient between the Pmsf and central venous pressure was unchanged [12].
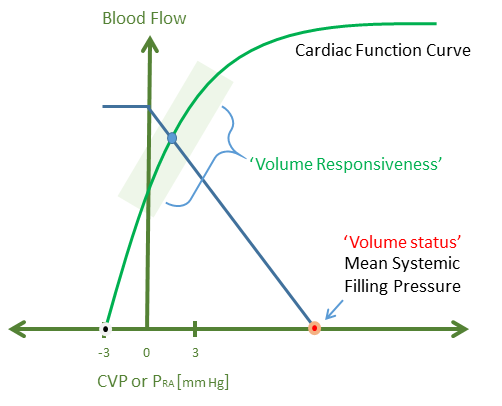
Crucially, these considerations raise the – often muddied – difference between volume status and volume responsiveness. As in part 1, volume status is but one portion of the Pmsf; yet the Pmsf cannot reveal whether the venous return curve intersects the ascending or plateau portion of the cardiac function curve – that is, speak for volume responsiveness - see figure 2.
Figure 2: A patient's 'volume status' is but one contributor to the mean systemic filling pressure on the x-axis. Volume responsiveness depends on where the venous return curve intersects the cardiac function curve. The x-axis is the central venous pressure or right atrial pressure and the y-axis is both venous return and cardiac output.
Implications for Echocardiography in Cardiac Arrest
Given the aforementioned discussion on Pmsf, it shouldn’t be surprising that a recent porcine model demonstrated RV dilation in the setting of hypovolemic cardiac arrest [13]. When heart function ceases, the right atrial and right ventricular pressure assume the Pmsf as circulatory pressures equilibrate. Thus, the Pmsf partially determines the distending pressure of the RV [the pressure surrounding the RV which tends to be negative at functional residual capacity also determines the RV transmural pressure].
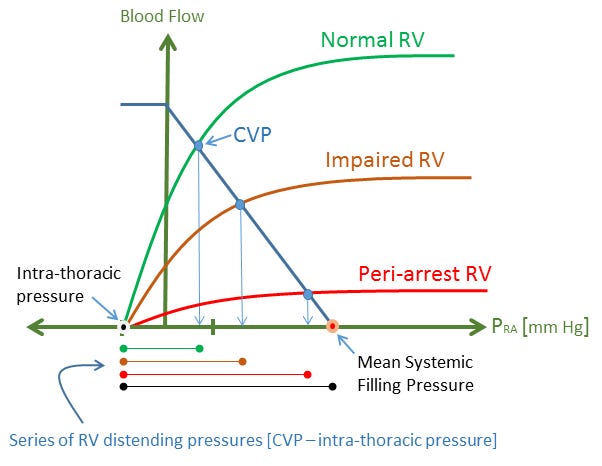
In the hypovolemic pigs, the right atrial pressure – and therefore the Pmsf – averaged 7 mmHg. While the ischemic RV is expected to stiffen somewhat, a distending pressure of 7 mmHg is still expected to provide a significant RV volume [14] because of the high compliance of the RV; this is of particular consequence in humans as Pinsky has shown that the RV normally operates as an un-stressed chamber [15]. Further, the Pmsf in the arrested circulation will rise in response to intrinsic and extrinsic adrenergic tone, for example, provision of epinephrine [11] - see figure 3.
Figure 3: Again, right atrial pressure is the x-axis and cardiac output and venous return are the y-axis. As the heart is progressively arrested, its function curve shifts down and the CVP eventually becomes the mean systemic filling pressure at cardiac standstill [a horizontal cardiac function curve]. The distending pressure of the RV is indicated below the graph and colour-coded for each state of the RV - black is cardiac arrest. Even in hypovolemic pigs during cardiac arrest, a mean systemic filling pressure of 7 mmHg is expected to provide a significant RV volume - thus an engorged RV is not surprising. During cardiac arrest, the mean systemic filling pressure - and therefore RV distending pressure - will increase in response to endogenous and exogenous catecholamines as described in part 1.
Please see other articles in this series,
References
Pinsky, M.R., Instantaneous venous return curves in an intact canine preparation. J Appl Physiol Respir Environ Exerc Physiol, 1984. 56(3): p. 765-71.
Maas, J.J., et al., Assessment of venous return curve and mean systemic filling pressure in postoperative cardiac surgery patients. Crit Care Med, 2009. 37(3): p. 912-8.
Maas, J.J., et al., Estimation of mean systemic filling pressure in postoperative cardiac surgery patients with three methods. Intensive Care Med, 2012. 38(9): p. 1452-1460.
Parkin, W.G. and M.S. Leaning, Therapeutic control of the circulation. J Clin Monit Comput, 2008. 22(6): p. 391-400.
Fessler, H.E., et al., Effects of Positive End-Expiratory Pressure on the Gradient for Venous Return. American Review of Respiratory Disease, 1991. 143(1): p. 19-24.
Fessler, H.E., Heart-lung interactions: applications in the critically ill. Eur Respir J, 1997. 10(1): p. 226-37.
Jellinek, H., et al., Influence of positive airway pressure on the pressure gradient for venous return in humans. J Appl Physiol (1985), 2000. 88(3): p. 926-32.
Luecke, T. and P. Pelosi, Clinical review: Positive end-expiratory pressure and cardiac output. Crit Care, 2005. 9(6): p. 607-21.
Pinsky, M.R., Determinants of pulmonary arterial flow variation during respiration. J Appl Physiol Respir Environ Exerc Physiol, 1984. 56(5): p. 1237-45.
Aya, H.D. and M. Cecconi, Can (and should) the venous tone be monitored at the bedside? Current opinion in critical care, 2015. 21(3): p. 240-244.
Funk, D.J., E. Jacobsohn, and A. Kumar, The role of venous return in critical illness and shock-part I: physiology. Crit Care Med, 2013. 41(1): p. 255-62.
Cecconi, M., et al., Changes in the mean systemic filling pressure during a fluid challenge in postsurgical intensive care patients. Intensive Care Med, 2013. 39(7): p. 1299-1305.
Aagaard, R., et al., The Right Ventricle Is Dilated During Resuscitation From Cardiac Arrest Caused by Hypovolemia: A Porcine Ultrasound Study. Crit Care Med, 2017.
Kuehne, T., et al., Combined pulmonary stenosis and insufficiency preserves myocardial contractility in the developing heart of growing swine at midterm follow-up. Journal of Applied Physiology, 2005. 99(4): p. 1422-1427.
Pinsky, M.R., My paper 20 years later: Effect of positive end-expiratory pressure on right ventricular function in humans. Intensive Care Med, 2014. 40(7): p. 935-41.