ICU Physiology in 1000 Words: Venous Excess & the Myth of Venous Return
Jon-Emile S. Kenny MD [@heart_lung]
In the last few weeks I have been contacted by curious clinical physiologists craving my conceptions of ‘venous excess’ [1]. These words will address this model, concisely and – I pray – clearly.
The Myth of Venous Return
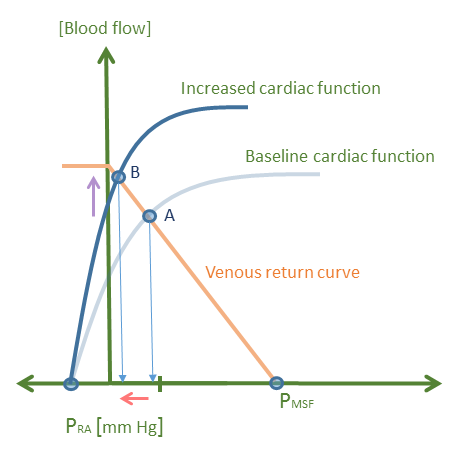
The roots of venous excess took hold within the fertile soil of a contrary interpretation of Guyton’s classic work on venous return [2]. Fear not, however, Guyton’s original curves have been independently-replicated, even with techniques different from his original experimental design [3-5]. The issue raised was not whether the relationship between flow from the venous beds and right atrial pressure was true; rather the concern was cause and effect – a chicken and egg argument, so to say – between right atrial pressure and blood flow [2]. It is often interpreted that – in the steady state – a fall in right atrial pressure ‘sucks’ in blood flow from the venules and veins. The critique, by contrast, is that it is the pump which is the primum movens of both cardiac output and venous return. Thus, it is entirely the heart distributing parcels of blood from the great veins to the great arteries which determine flow and the observed fall in right atrial pressure is simply an epiphenomenon of the heart’s action. Accordingly, per this critique, in the steady state, there is a functional relationship between blood flow from the periphery and right atrial pressure that is perfectly described by Guyton’s curve, but the fall in right atrial pressure and rise in venous return is not cause and effect [see figure 1].
Figure 1: An increase in cardiac function from point A to point B. Note that right atrial pressure [x-axis] falls - red arrow and blood flow [cardiac output or venous return] increases on y-axis [purple arrow]. The question is: does venous return increase because right atrial pressure falls, or does blood flow increase and right atrial pressure fall as a consequence? Pra is right atrial pressure, Pmsf is mean systemic filling pressure. Venous return curve is light orange, cardiac function curves are blue.
A critical reappraisal is offered via an independent experiment [5] where the relationship between blood flow and right atrial pressure was assessed but with right atrial pressure – the dependent variable – on the y-axis, and flow – the independent variable – on the x-axis; in other words, a ‘flipped’ Guyton curve [2]. This depiction is more mathematically precise in that the right atrial pressure, or the central venous pressure, is ultimately the dependent variable, dancing in response to changes in vascular and cardiac function.
A Criticism of a Criticism
Whether or not the above is true has previously been litigated by physiologists of great scientific stature [6-8]; Guyton himself considered the argument unhelpfully circular [1, 9]. My concern with the aforementioned really distills down to a critical distinction between transient and steady-state effects. Indeed, it is clearly highlighted in the critical analysis above [2] - see pg. 851 - that the arguments put forth apply only to the vasculature when it is in the steady state, i.e. when:
volume contained within the peripheral vasculature is constant
that regional compliances do not change,
that flow is not redistributed among the parallel organ vasculatures
that no other energy sources [muscle pumping, respiratory pumping] are present [2].
Upon reviewing the aforementioned conditions it is unmistakable how rare it is to find a patient in the operating theatre or intensive care unit in the steady state. Nearly every intervention [e.g. fluid bolus, vasoactive infusion, dynamic shock, change in intra-thoracic pressure/breathing, etc.] violates theses constraints rendering the criticisms described at the outset as clinically moot. It is therefore critical that ‘venous return’ not be brushed aside as a ‘myth’ based on an argument valid only during a highly controlled experimental paradigm. When a litre of saline is infused into a peripheral vein, when a deep inspiratory effort is made, both the up [i.e. mean systemic filling pressure], and down [i.e. right atrial] - stream pressures of venous return are real, important and the primum movens of flow. Thus venous return must be understood by students as a true and separate – albeit transient – entity from cardiac output. This is supported by our collective experiences, empirical evidence [6, 10-13] and this pretty ultrasound.
Venous Excess
The idea of venous excess as a ‘tangible’ cardiovascular variable grew forth from the short-comings of venous return described above; that is, venous return is not distinct from cardiac output and that right atrial pressure does not have a causative role in blood flow [1]. Accordingly, it is argued that the equation that describes venous return as an upstream and downstream pressure is too nebulous for medical students to comprehend. For instance, the authors argue that mean systemic filling pressure is unhelpful because it cannot be measured and it cannot be physically located within the venous system [1].
Rather than being a valid scientific argument against the mean systemic filling pressure, it strikes me rather as venous xenophobia. To dismiss a principle or concept because it does not fit within the norms of what one considers ‘real’ on the arterial side of the circulation lays bare a sad vascular relativism. Ask the cocksure physiologist to pinpoint exactly 'where' – anatomically – the mean arterial pressure lies and the certainty and ‘realness’ of a readily measured variable becomes less incontrovertible. Let this not be the arbiter of what is worthy of physiological consideration; equally, let us not be fooled into thinking that what can be measured is facilely understood.
The proponents of venous excess also argue that venous return ignores the importance of venous capacitance; but it does not. Venous capacitance is an integral part of the mean systemic filling pressure – the ‘upstream’ pressure for flow returning to the heart [14-19]. A low venous capacitance indicates a higher venous pressure for a given volume, while a high venous capacitance is just the opposite.
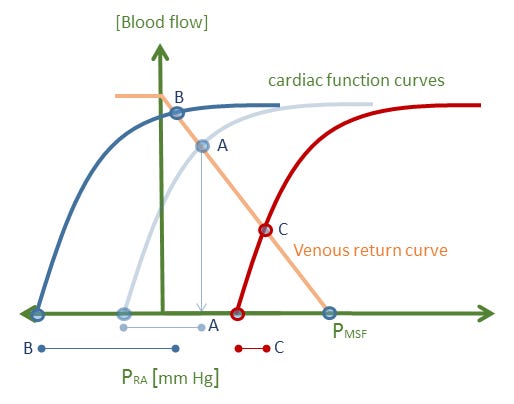
The idea of venous excess itself, however, is a good one – in a sense providing a measure of ‘stretch’ in the great veins and right heart. This may then act as an ‘error signal’ for example, triggering the release of atrial natriuretic peptide if stretch is too great [1]. But ‘venous excess’ is much better gleaned from a Guytonian analysis – as the transmural right atrial pressure [see figure 2]. The importance of using a Guyton diagram to illustrate venous excess is apparent when one considers the super-position of the respiratory pump [e.g. deep inspirations, application of PEEP]. When the intra-thoracic pressure changes, there is a dissociation between the pressures across the right heart [e.g. right atrial pressure relative to the pleural pressure] from the pressure within the right heart. This discrepancy is easily visualized on the Guyton diagram and is a superior way to describe ‘venous excess’ to students, who may speciously equate an increase in absolute right atrial pressure with an increase in its distending pressure.
Figure 2: The effect of intra-thoracic pressure on right heart trans-mural pressure. Baseline is state A in light blue. The right atrial pressure is marked point A, the transmural pressure is the faint blue dumbbell below the graphic. The intra-thoracic [or surrounding] pressure is estimated as the x-intercept of the cardiac function curve. Point B represents a deep inspiration; note that right atrial pressure has fallen, but that the transmural pressure [or venous excess] has increased as marked by the dark blue dumbbell below the x-axis. By contrast, the red cardiac function curve represents an increase in intra-thoracic pressure. While the measured right atrial pressure has increased to point C, the transmural pressure [venous excess] has fallen as indicated by the red dumbbell below the x-axis. This graphic assumes no change in vascular capacitance with change in intra-thoracic pressure for diagram simplicity.
Please see more posts in this series,
References
Reddi, B. and R. Carpenter, Venous excess: a new approach to cardiovascular control and its teaching. J Appl Physiol, 2005. 98(1): p. 356-364.
Brengelmann, G.L., A critical analysis of the view that right atrial pressure determines venous return. J Appl Physiol, 2003. 94(3): p. 849-859.
Greene, A.S. and A.A. Shoukas, Changes in canine cardiac function and venous return curves by the carotid baroreflex. American Journal of Physiology-Heart and Circulatory Physiology, 1986. 251(2): p. H288-H296.
Hatanaka, T., J.T. Potts, and A.A. Shoukas, Invariance of the resistance to venous return to carotid sinus baroreflex control. American Journal of Physiology-Heart and Circulatory Physiology, 1996. 271(3): p. H1022-H1030.
Levy, M.N., The cardiac and vascular factors that determine systemic blood flow. Circ Res, 1979. 44(6): p. 739-747.
Magder, S., Point: Counterpoint: The classical Guyton view that mean systemic pressure, right atrial pressure, and venous resistance govern venous return is/is not correct. J Appl Physiol, 2006. 101(5): p. 1523-1525.
Brengelmann, G., Counterpoint: the classical Guyton view that mean systemic pressure, right atrial pressure, and venous resistance govern venous return is not correct. J Appl Physiol, 2006. 101(5): p. 1525-1526.
Pinsky, M.R., The classical Guyton view that mean systemic pressure, right atrial pressure, and venous resistance govern venous return is/is not correct. J Appl Physiol, 2006. 101(5): p. 1528-1530.
Guyton, A.C. and T. CE Coleman, Circulatory physiology: cardiac output and its regulation. 1973.
Deschamps, A. and S. Magder, Baroreflex control of regional capacitance and blood flow distribution with or without alpha-adrenergic blockade. American Journal of Physiology-Heart and Circulatory Physiology, 1992. 263(6): p. H1755-H1763.
Deschamps, A., A. Fournier, and S. Magder, Influence of neuropeptide Y on regional vascular capacitance in dogs. American Journal of Physiology-Heart and Circulatory Physiology, 1994. 266(1): p. H165-H170.
Deschamps, A. and S. Magder, Effects of heat stress on vascular capacitance. American Journal of Physiology-Heart and Circulatory Physiology, 1994. 266(5): p. H2122-H2129.
Mitzner, W. and H. Goldberg, Effects of epinephrine on resisitive and compliant properties of the canine vasculature. J Appl Physiol, 1975. 39(2): p. 272-280.
Rothe, C.F., Physiology of venous return. An unappreciated boost to the heart. Arch Intern Med, 1986. 146(5): p. 977-82.
Gelman, S., Venous function and central venous pressure: a physiologic story. Anesthesiology, 2008. 108(4): p. 735-48.
Henderson, W.R., et al., Clinical review: Guyton--the role of mean circulatory filling pressure and right atrial pressure in controlling cardiac output. Crit Care, 2010. 14(6): p. 243.
Funk, D.J., E. Jacobsohn, and A. Kumar, The role of venous return in critical illness and shock-part I: physiology. Crit Care Med, 2013. 41(1): p. 255-62.
Funk, D.J., E. Jacobsohn, and A. Kumar, Role of the venous return in critical illness and shock: part II-shock and mechanical ventilation. Crit Care Med, 2013. 41(2): p. 573-9.
Jacobsohn, E., R. Chorn, and M. OConnor, The role of the vasculature in regulating venous return and cardiac output: historical and graphical approach. Canadian Journal of Anaesthesia-Journal Canadien D Anesthesie, 1997. 44(8): p. 849-867.