ICU Physiology in 1000 Words: Pulmonary Embolism & Pulmonary Vascular Resistance
Jon-Emile S. Kenny MD [@heart_lung]
What if I told you that in acute pulmonary thromboembolism [PE] that the initiation of intravenous norepinephrine [NE] decreases the calculated pulmonary vascular resistance [cPVR]? Would you believe me? Certainly, you would trust that an infusion of a thrombolytic does so. Of great interest, both NE and thrombolytics decrease the cPVR in acute PE [1-7]. But why is this? Could it be that NE induces the pulmonary circulation to open itself like an accordion – with an airy sigh and harmonic hum of blood stirring swiftly through dilated vascular bellows?
Or might the cPVR be fooling us?
Perhaps the concord of sounds we hear across the lungs is not driven by a dilating squeeze box, but rather express the synthetic sounds of simplified physiological assumptions? If you have read my previous ramblings on this topic, you might know how this song will conclude. By the end, I hope to show how both NE and thrombolysis lower cPVR in acute PE, but how only the latter truly lowers the lifted load by the right ventricle [RV].
Basics
This analysis will require interpreting pulmonary artery pressure-flow [PQ] relationships [1, 2, 8]. These are graphical representations of the ‘pulmonary vascular resistance’ in its broadest sense. The x-axis is blood flow [Q – cardiac output, the independent variable] and the y-axis is mean pulmonary artery pressure [P – the dependent variable]. Thus, the slope of a line on the PQ plot represents the change in P [in the numerator] for a given change in Q [in the denominator]; this is the calculated pulmonary vascular resistance [see figure 1 bottom].
Thought Experiment
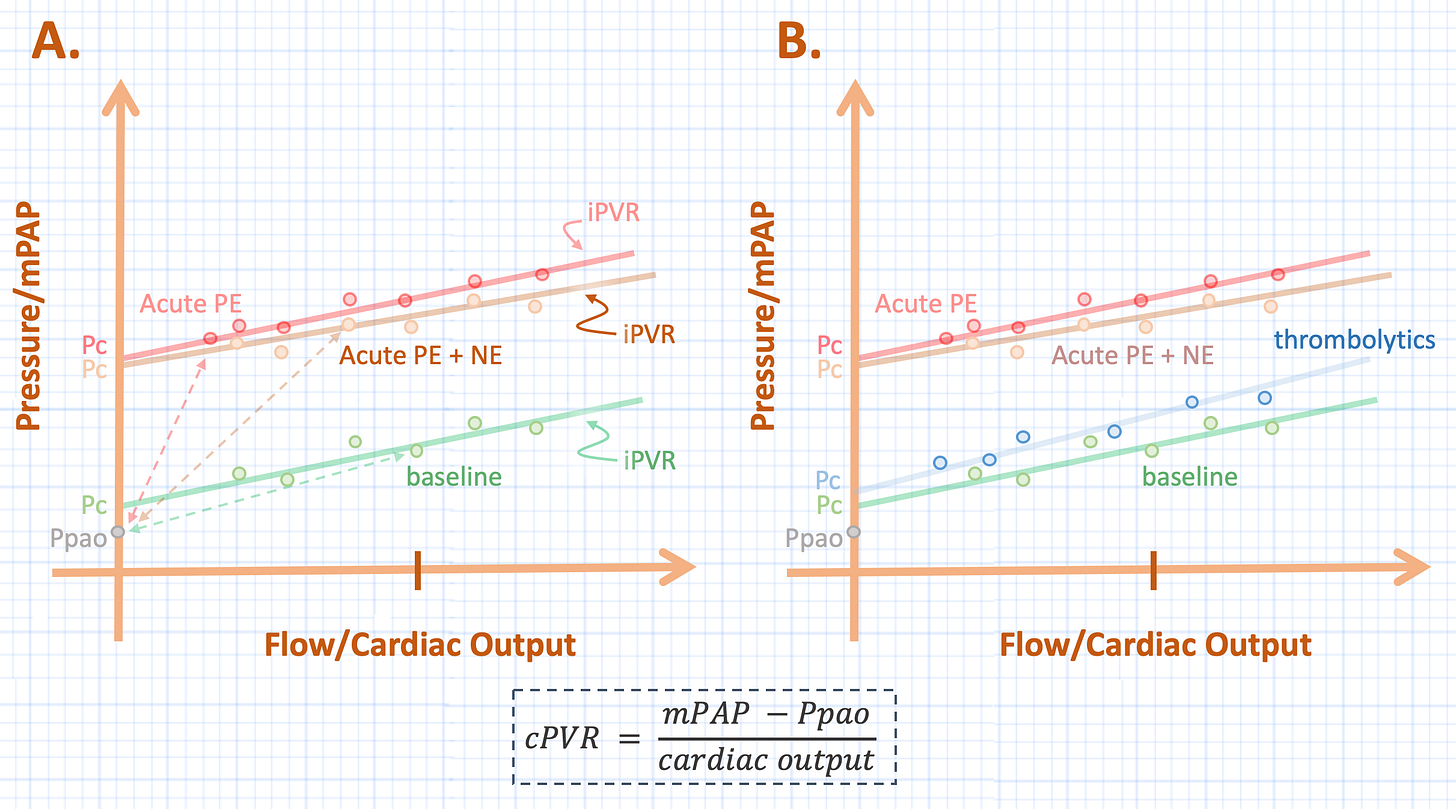
Imagine that a patient has a normal mean pulmonary artery pressure [mPAP] at a normal cardiac output for a given pulmonary artery occlusion pressure [Ppao or ‘wedge pressure’]; this value is the green point on the PQ plot in figure 1A. The slope of the line connecting the Ppao to the mPAP is the cPVR at this point [green dotted line, see equation].
Figure 1: A - shows the calculated pulmonary vascular resistance [cPVR] [slope of dotted line] at baseline [green], following PE [red] with subsequent infusion of NE [orange]. B - shows extrapolated PQ plots for baseline [green] and following PE [red]. See text for details. Pc is closing pressure, Ppao is pulmonary artery occlusion pressure, mPAP is mean pulmonary artery pressure, iPVR is incremental pulmonary vascular resistance. Compare the iPVR to the cPVR in B.
Now imagine that this unfortunate patient suffers a very large PE. The mPAP rises and the cardiac output falls as depicted by the red point in figure 1A. Assuming a constant Ppao, it is easily visualized that the slope of the line connecting the mPAP to the Ppao has risen; that is, the cPVR has increased [red dotted line]. In other words, for a given change in flow on the x-axis, the change in pressure on the y-axis is greater.
Finally, imagine initiating a NE infusion. The alpha effects of the drug improve the gradient for venous return [9], stimulating the Frank-Starling mechanism and the mild inotropic effects increase the contractility of the heart. Accordingly, blood flow along the x-axis increases and the mPAP climbs as more blood fills the pulmonary circuit. This is illustrated by the orange point in figure 1A and the dotted line connecting it to the constant Ppao demonstrates that the cPVR has decreased; in other words, as compared to the cPVR during acute PE [red dotted line], the change in pressure for a given change in blood flow is less with NE [orange dotted line].
While the aforementioned is hypothetical, this physiology has been demonstrated in multiple animal models of acute PE treated with NE [1-5]; I have not made this up!
The Caveat
The rub is that the Ppao is often not the downstream pressure in the pulmonary circuit and certainly not so during acute PE. The downstream pressure is actually a ‘closing pressure’ [Pc] that must be overcome before flow occurs, and it is typically greater than the Ppao. The reason for this can be modeled using ‘Starling Resistors’; an example is the Zones of West in the lung. If a particular portion of the lung has a trans-pulmonary pressure [Ptp, alveolar pressure minus the pleural pressure] of 15 mmHg across it, while another section has a Ptp of only 8 mmHg and the Ppao is below both at 5 mmHg, then the closing pressure [i.e. the pressure above which flow occurs, Pc] for the total lung will be a conductance weighted-average of each lung section – illustrated mathematically by Mitzner many decades ago [10, 11].
A way to measure the Pc of the pulmonary circulation is to measure multiple pressure-flow coordinates at a stable Ppao and extrapolate the line to the y-intercept of the PQ plot [2]. The Pc is typically slightly higher than the Ppao at most physiological values, but as the Ppao rises, the closing pressure and Ppao begin to equalize. In healthy dogs, this occurs at a Ppao of roughly 19 mmHg [11].
PE, NE & the PQ
How does an acute PE affect the PQ plot? Its most notable consequence is that is greatly increases the closing pressure of the pulmonary circulation, with minimal effect on the slope of the PQ plot [see figure 1B] [1, 2, 4, 12]. The slope of the line from the mPAP to the closing pressure has been coined the ‘incremental’ pulmonary vascular resistance [iPVR] to distinguish is from the cPVR, which is referenced to the Ppao. Thus PE, raises the Pc with minimal effect on the iPVR.
And what happens when NE is added to the mix? When a whole PQ plot is generated for a given dose of NE in PE, it lines up almost entirely upon the slope of the PE without NE [4]. Accordingly, NE does not alter the iPVR [see figure 2A]. The problem with the cPVR can be easily seen graphically; when compared to the cPVR of acute PE [red dotted line], the slope of the orange dotted line [cPVR for the addition of NE] falls if referenced to the Ppao. Yet, when correctly referenced to its Pc, there is minimal difference between acute PE and the addition of NE.
Figure 2: A - shows the effect of NE infusion in PE using a full PQ plot, compare the iPVR [solid line] to cPVR [dotted line] during PE [red] and the addition of NE [orange]. B - shows the effect of tPA [blue]. See text for details. Pc is closing pressure, Ppao is pulmonary artery occlusion pressure, mPAP is mean pulmonary artery pressure, iPVR is incremental pulmonary vascular resistance.
Thrombolysis
As anticipated, the effect of thrombolytics is to lower the closing pressure back to its baseline state following an acute PE [2, 6]. Lytics change the iPVR slightly, but its greatest benefit to the RV is by lowering the Pc – akin to ‘opening’ a very large Starling Resistor [13] [see figure 2B].
Alas, the dissonance of the cPVR still grates. With time, I pray, non-invasive measures of RV-pulmonary artery coupling [14] will ring truth like a warm, struck bell. Yea, send not to know for whom the bell tolls; cPVR, it tolls for thee.
Please see other posts in this series,
JE
Dr. Kenny is the cofounder and Chief Medical Officer of Flosonics Medical; he also the creator and author of a free hemodynamic curriculum at heart-lung.org
References
Ducas J, Prewitt R: Pathophysiology and therapy of right ventricular dysfunction due to pulmonary embolism. Cardiovascular clinics 1987, 17(2):191-202.
Prewitt RM: Hemodynamic management in pulmonary embolism and acute hypoxemic respiratory failure. Critical care medicine 1990, 18(1 Pt 2):S61-69.
Molloy WD, Lee K, Girling L et al: Treatment of shock in a canine model of pulmonary embolism. American Review of Respiratory Disease 1984, 130(5):870-874.
Ducas J, Duval D, Dasilva H et al: Treatment of canine pulmonary hypertension: effects of norepinephrine and isoproterenol on pulmonary vascular pressure-flow characteristics. Circulation 1987, 75(1):235-242.
Ghignone M, Girling L, Prewitt R: Volume expansion versus norepinephrine in treatment of a low cardiac output complicating an acute increase in right ventricular afterload in dogs. Anesthesiology 1984, 60(2):132-135.
Prewitt RM, Shiffman F, Greenberg D et al: Recombinant tissue-type plasminogen activator in canine embolic pulmonary hypertension. Effects of bolus versus short-term administration on dynamics of thrombolysis and on pulmonary vascular pressure-flow characteristics. Circulation 1989, 79(4):929-938.
Angle MR, Molloy DW, Penner B et al: The cardiopulmonary and renal hemodynamic effects of norepinephrine in canine pulmonary embolism. Chest 1989, 95(6):1333-1337.
Layish DT, Tapson VF: Pharmacologic hemodynamic support in massive pulmonary embolism. Chest 1997, 111(1):218-224.
Wood KE: Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest 2002, 121(3):877-905.
Mitzner WA: Resistance of the pulmonary circulation. Clinics in chest medicine 1983, 4(2):127-137.
Ducas J, Schick U, Girling L et al: Effects of altered left atrial pressure on pulmonary vascular pressure-flow relationships. American Journal of Physiology-Heart and Circulatory Physiology 1988, 255(1):H19-H25.
Duval D, Ducas J, Molloy W et al: Effects of pulmonary (P) emboli (E) and noradrenaline (NE) on pulmonary pressure-flow relationships. Am Rev Respir Dis 1984, 129(4):A63.
Lopez-Muniz R, Stephens N, Bromberger-Barnea B et al: Critical closure of pulmonary vessels analyzed in terms of Starling resistor model. Journal of Applied Physiology 1968, 24(5):625-635.
Kerbaul F, Rondelet B, Motte S et al: Effects of norepinephrine and dobutamine on pressure load-induced right ventricular failure. Critical care medicine 2004, 32(4):1035-1040.