ICU Physiology in 1000 Words: Airway Pressure Release Ventilation – Part 3
Jon-Emile S. Kenny MD [@heart_lung]
Lung Stress in Pulmonary & Extrapulmonary ARDS
Initially described in the late 1990s, the distinction between direct pulmonary insults [i.e. pulmonary ARDS] and indirect pulmonary insults [i.e. extra-pulmonary ARDS] is important [1]. Additionally, direct pulmonary injury such as gastric acid aspiration may have a different molecular phenotype from indirect, extra-pulmonary affronts such as trauma or pancreatitis. Yet, for this discussion, the original, mechanical distinction is key; i.e. the proportional differences in lung stress relative to total respiratory system stress.
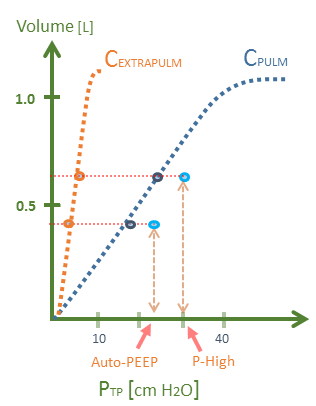
The stiffness of the lungs and chest wall together may be quantified as the elastance of the respiratory system [Ers], while the stiffness of only the lungs is lung elastance [El]. In pulmonary ARDS, the ratio of the lung stiffness to the total respiratory system stiffness is high – upwards of 80% [El/Ers = 0.8] [2, 3]. By contrast, in extra-pulmonary ARDS, the relationship between lung stiffness and total system stiffness is low. This value can be as low as 20% [El/Ers = 0.2]. The El/Etot ratio is important for when it is multiplied by the change in pressure applied at the airway [i.e. the driving pressure], the change in trans-pulmonary pressure [Ptp] – or stress – across the lung is given. As well, if one assumes that a breath begins with a pleural pressure close to zero [as it can be in the upper, non-dependent lung], then multiplying the airway pressure by the El/Etot approximates the Ptp.
Figure 1: A hypothetical D-type breath in P-APRV. P-High of 30 cm H2O could result in a lung stress [Ptp] of 18 to 24 cm H2O in pulmonary ARDS [blue curve] compared to a stress [Ptp] of only 4.5 to 6 cm H2O in extra-pulmonary ARDS or obesity [orange curve]. Note x-axis is Ptp [transpulmonary pressure or whole lung stress], y-axis is volume in litres. The floating light blue circles represent the airway pressure. Assumptions - respiratory system compliance is 20 mL/cmH2O, EF-PEFR-75%, P-Low is zero. El/Etot is 0.2 for extra-pulm, El/Etot is 0.8 for pulm ARDS, breath begins with Ppl of 0 cm H2O.
Knowing the Ptp during a D-breath at P-High is essential because it partially defines the envelope of the area under the pressure-volume curve – or work – applied to the lung by the ventilator [3]. Critically, in a recent trial comparing APRV to conventional, low tidal volume ventilation [LTV] in ARDS, an apparent failure of randomization allocated a disproportionate number of patients with extra-pulmonary ARDS to APRV [only 25% had direct causes]; by contrast, 39% had pulmonary or direct causes of ARDS in the LTV group [4]. Therefore, the APRV patients in this trial experienced less lung stress at any given airway pressure; accordingly, the favourable APRV findings – in my opinion – are tenuous.
Ergotrauma in APRV – a theoretical model
As previously described, the work performed on the lung during a single breath is divided into dynamic [e.g. gas flow] and static components [e.g. lung volume]. The summation of the dynamic and static work over time determines the mechanical power – in Joules per minute – applied to the lung [3, 5]. Thus, VILI secondary to mechanical power – ergotrauma – provides a powerful unification of multiple avenues of VILI research [2]; neither static pressure nor volume alone drive VILI, but rather, baseline stretch [i.e. PEEP, auto-PEEP] as well as airway resistance, gas flow and respiratory rate are all mediators. Augmenting any of these variables boosts the power applied to the lung skeleton and, in theory, VILI risk. But how does this relate to P-APRV?
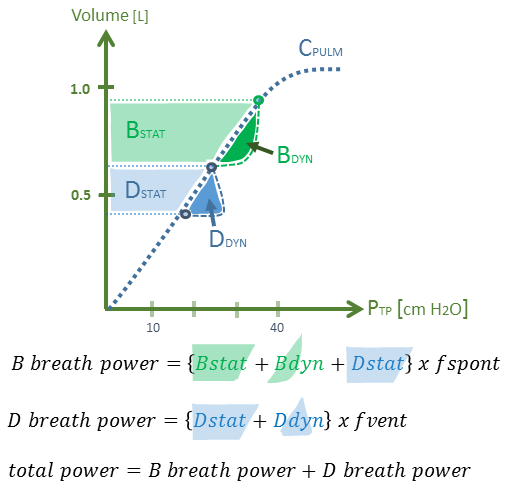
Firstly, the static work of the D-type breaths [Dstat], is defined by the envelope between the auto-PEEP generated at T-Low, and the Ptp generated at P-High [figure 2A & B]. But there is also a dynamic component to a D-type breath [i.e. Ddyn] that is described as gas flow multiplied by airway resistance. This parcel is illustrated to the right of the lung elastance curve. Notably, a D-breath in APRV demands gas volume delivered over a very short period of time – that is, a high inspiratory flow. Gas supplied swiftly supports turbulent flow which increases effective resistance and, therefore, dynamic work. Indeed, high flow rates are a known determinant of VILI [5].
Figure 2A: Theoretical model for P-APRV power calculation in pulmonary ARDS. The area of the dynamic and static envelopes is the work for a single breath type [D versus B], but the power is solved by this area multiplied by its frequency of application to the lung. Dstat & Ddyn are static & dynamic work of breathing for D breath, respectively. Bstat & Bdyn are static & dynamic WOB for B breath, respectively. f-spont is spontaneous respiratory rate. f-vent = 60 / T-High (sec) + T-Low (sec). x-axis is Ptp or transpulmonary pressure while y-axis is volume in litres.*
Secondly, B-type breaths must also be considered in the power calculation [see figures 2A & B]. Because B-breaths occur at P-High, they increase Ptp and lung volume beyond that of the D-breath. This additional work allotment, like a D breath, comprises static and dynamic elements. Ostensibly, the dynamic work is reduced at higher lung volume given tethering of airways. Yet, like the D-breath, the static and dynamic parcels of work must be multiplied by their rate to determine joules/minute, or mechanical power. Crucially, the total static work for each spontaneous B-breath includes the static work that is generated when the lung is placed at a higher volume by the D-breath; why? Using Hook’s Law as an analogy [2], consider a malleable material stretched from its baseline length (l) [e.g. (l) + x] and then extended repeatedly beyond x [i.e. repetitions between x and higher length y]. The total power applied is [(l) + y] multiplied by the stretch frequency. Thus, the work for a single B-breath is the static and dynamic work of the B-breath multiplied by the patient’s spontaneous respiratory rate. From this analysis it is understood that the power applied to the directly-insulted lung can be very large in P-APRV.
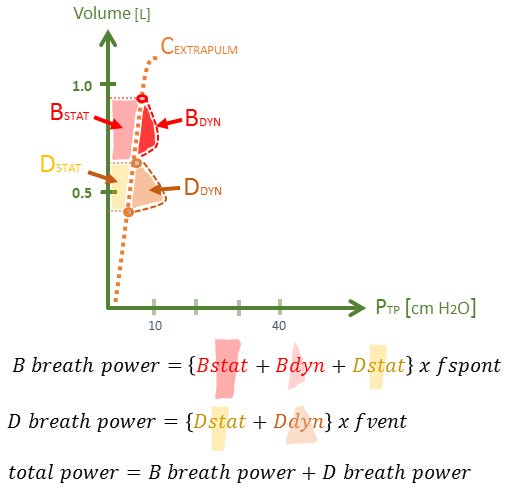
Figure 2B: theoretical power calculation for P-ARPV in extra-pulmonary ARDS, compare work & power to that of 2A for pulmonary or direct ARDS. In the recent Zhou et al. trial [4], considerably more patients randomized to APRV may have had this physiology [i.e. extra-pulmonary ARDS] and therefore lower power. Please note error in this figure; B breath power does not include Dstat work.
Empirical Evidence
There is sparse data for stress across the lung during APRV let alone mechanical power. In one report, a patient receiving F-APRV [with a relatively long P-Low] engaged in mostly D and B breaths – similar to what one would expect during P-ARPV [6]. Importantly, an esophageal balloon was placed so both an approximation of lung stress [i.e. Ptp] was tracked with lung strain [i.e. lung volume]. Close inspection of the airway pressure curve relative to esophageal pressure [Pes] curve at the end of D and B-breaths reveals that both Ptp and lung volume doubled during a B-breath. This is consistent with increased stress and strain during spontaneous breathing in APRV [6, 7].
Work of breathing [WOB] in joules per litre during APRV has been calculated in a small cohort of patients receiving F-APRV [8]. One patient in this investigation – ‘subject 9’ – engaged in mostly B and D type breaths. Here, spontaneous WOB was 1.25 joules/Litre while the ventilator delivered roughly 2.75 Joules/Litre of work for a total of roughly 4.0 J/L [recall that a normal work of breathing is 0.3 to 0.6 J/L [9]]. As minute ventilation was 12 L/min, the overall power applied to the lung was 48 joules/minute! The power threshold for VILI is not known in humans; in a swine model, it was 12 J/min [10]. As human lungs have twice the specific elastance as swine [11], one would expect this threshold to at least double in humans, but these comparisons are extremely tenuous.
Caveat and Conclusion
An intriguing investigation demonstrated that the fall in pleural pressure [Ppl] during spontaneous effort is not distributed evenly across all pleural surfaces in ARDS – as it does in health [12]. Rather, the lower lobes experience disproportionately low Ppl leading to cryptic stress, strain and pendelluft – sloshing of air from the ventral to the dorsal lung units at the onset of inspiration. Crucially, the Ppl observed was much lower than the Pes; accordingly, the aforementioned calculations may actually underestimate the total power during B-type breaths. Nevertheless, the high auto-PEEP generated by APRV may diminish lower lobe stress and strain during spontaneous breathing [13].
Thus, widely opening the lung during inspiration and expiration reduces microstrain, but the pressure required can multiply the mechanical power experienced by the lung – especially if supplemented by spontaneous breaths. Evidence for aggressive recruitment – an attempt to reduce lung inhomogeneity and stress raisers – is revealing a cryptic cost. Should we adopt a lower threshold for prone position – which homogenizes stress raisers without increased power – than for initiation of APRV? Finally, should we be open to closing the lung and keeping it closed [14]?
*In a previous version of this post, I argued that the energy of a B-breath included the static energy of a D-breath; on reflection, I no longer think this is accurate. In other words, the power of a B-breath is simply (B-stat + B-dyn) x fspont [see figure 2A and B]
Please check out other posts in the ‘1000 Word’ series,
JE
Dr. Kenny is the cofounder and Chief Medical Officer of Flosonics Medical; he is also the creator and author of a free hemodynamic curriculum at heart-lung.org
References
Gattinoni, L., et al., Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease: different syndromes? American journal of respiratory and critical care medicine, 1998. 158(1): p. 3-11.
Tonetti, T., et al., Driving pressure and mechanical power: new targets for VILI prevention. Annals of translational medicine, 2017. 5(14).
Gattinoni, L., et al., The future of mechanical ventilation: lessons from the present and the past. Critical Care, 2017. 21(1): p. 183.
Zhou, Y., et al., Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome. Intensive care medicine, 2017. 43(11): p. 1648-1659.
Marini, J.J. and S. Jaber, Dynamic predictors of VILI risk: beyond the driving pressure, 2016, Springer.
Neumann, P., et al., Influence of different release times on spontaneous breathing pattern during airway pressure release ventilation. Intensive care medicine, 2002. 28(12): p. 1742-1749.
Mireles-Cabodevila, E. and R.M. Kacmarek, Should airway pressure release ventilation be the primary mode in ARDS? Respiratory care, 2016. 61(6): p. 761-773.
Kallet, R.H., Patient-ventilator interaction during acute lung injury, and the role of spontaneous breathing: part 2: airway pressure release ventilation. Respiratory care, 2011. 56(2): p. 190-206.
Banner, M.J., M.J. Jaeger, and R.R. Kirby, Components of the work of breathing and implications for monitoring ventilator-dependent patients. Critical care medicine, 1994. 22(3): p. 515-523.
Cressoni, M., et al., Mechanical power and development of ventilator-induced lung injury. Anesthesiology: The Journal of the American Society of Anesthesiologists, 2016. 124(5): p. 1100-1108.
Protti, A., et al., Lung stress and strain during mechanical ventilation: any safe threshold? American journal of respiratory and critical care medicine, 2011. 183(10): p. 1354-1362.
Yoshida, T., et al., Spontaneous effort causes occult pendelluft during mechanical ventilation. American journal of respiratory and critical care medicine, 2013. 188(12): p. 1420-1427.
Morais, C.C., et al., High Positive End-Expiratory Pressure Renders Spontaneous Effort Non-Injurious. American journal of respiratory and critical care medicine, 2018; May 15.
Pelosi, P., P.R.M. Rocco, and M.G. de Abreu, Close down the lungs and keep them resting to minimize ventilator-induced lung injury. Critical Care, 2018. 22(1): p. 72.