ICU Physiology in 1000 Words: The Venous Excess Ultrasound Score Is Not the Mean Systemic Pressure
Jon-Emile S. Kenny MD [@heart_lung]
Since its first description [1], the Venous Excess Ultrasound Score [VExUS] has received much research attention. Very simply, VExUS combines the size of the inferior cava [IVC] with Doppler patterns from the hepatic, portal and intrarenal veins. From these measures a score from 0 to 3 is given as a marker of increasing ‘venous congestion [VC].’ But what exactly does VC mean? And perhaps more importantly, once we know its meaning, what does one do about it?
Synonyms for the adjective ‘congested’ are: ‘overfull,’ ‘overflowing,’ ‘overloaded,’ and ‘clogged.’ Therefore, ‘congestion’ is a clinically-loaded term; it is understandable that we might reflexively link a congested state with ‘volume overload’ and reach for a diuretic. But should we? It follows that we might want to relate the VExUS score to a measure of ‘volume status’, even though this nebulous term is challenging to triangulate. But should we?
Beyond radiolabeled albumin studies [2], one potential objective measure of volume state is the mean systemic filling pressure [Pmsf] [3-5]. The Pmsf is the pressure within the systemic circulation when blood flow stops; it is the x-intercept of the venous return curve and it is directly, though imperfectly, related to volume state as described by Rothe [4, 5]. Might the Pmsf relate to VExUS? This is a question that has been asked at least once [6, 7]. However, first principles should temper our enthusiasm; there is little relationship betwixt Pmsf and VExUS!
VExUS and Pra
While VExUS is a relatively new paradigm, early studies clearly relate the undulating venous flow morphology to the right atrial pressure [Pra] trace. Some of the earliest work in this realm was conducted on veins not included in the VExUS score – the superior vena cava [SVC] and internal jugular vein [IJV]. In 1954 Brecher [8], using a ‘bristle flowmeter’ wrapped around the SVC, observed that systolic [S-wave] and diastolic [D-wave] waves occurred in step with the x’ and y descents of the central venous pressure trace, respectively [Figure 1A]. Subsequently in the late 1970s, Sivaciyan and Ranganathan [9] confirmed these findings with Doppler ultrasound of the IJV. They noted that with rising Pra and right heart dysfunction, the S-wave in the jugular vein shrinks relative to the D-wave. Furthermore, in the patients who had invasive hemodynamic measures and in whom they found diastolic-only IJV filling, 100% had abnormal right heart function.
Changes in the Pra trace, as described above, are also observed in the inferior vena cava [10], femoral [11], hepatic [12], portal [13] and intrarenal veins [14, 15]. While the latter 3 vessels comprise VExUS, the physiology between all the veins are largely interchangeable. Indeed, any review or description of VExUS contains at least a cursory description of the Pra trace [16]. Given all this, it is very reasonable to presume that, in general, venous Doppler – and VExUS more specifically – are all surrogates of Pra and/or right heart hemodynamics. To be sure, two recent evaluations found that a direct relationship between VExUS and Pra [17, 18].
Pmsf and Pra
If VExUS speaks to us through the Pra, how does Pra relate to Pmsf? This is immediately gleaned via the equation of Parkin and Leaning [3, 19].
This equation shows that there is a direct relationship between Pmsf [i.e., the mean systemic pressure analogue, Pmsa] and Pra. In other words, if Pra rises – or by extension, VExUS – so too will Pmsa and vice versa. However, Pmsa is also mediated by cardiac output and MAP. Thus, if Pra and CO are rising together, then there will be a rough, positive correlation between Pmsa and Pra [Figure 1B]
However, what happens when Pra and CO move in opposite directions? For example, when there is progressively worsening cardiac function, CO falls and Pra rises [Figure 1C]. Here, the Pmsa might not change much at all, even with a rising Pra [i.e., rising venous congestion]. Hypothetically, if one could plot all possible Pmsa-Pra combinations, there would be little-to-no relationship between Pra and Pmsa; plotting all potential Pmsa-VExUS permutations would yield similar results.
Figure 1: VExUS, Pra and Pmsa. A.) Illustration of venous Doppler trace in relation to right atrial pressure trace. B.) illustrates what happens when Pra and CO rise together, caused by increasing Pmsa; this could be the result of a fluid bolus [i.e., Pmsa1-3]. The result is that the Pmsa also increases such that Pmsa and Pra correlate [inset, upper right]. C.) illustrates what happens when Pra rises and CO falls, this could be due to worsening cardiac function [i.e., Rcardiac 1-3], for example from a worsening pulmonary embolism. Pmsa and Pra do not correlate [inset, upper right]. OP is operating point, the operating point defines the cardiac output on the y-axis and Pra on the x-axis; The red lines are Rcardiac or cardiac resistance [i.e., cardiac function]. The blue lines represent venous return. Ppc is pericardial pressure.
Key Caveat: the transmural Pra
One key consideration when relating VExUS to Pra – and therefore Pmsa – is pressure measurement accuracy [20]. Whenever measuring the Pra, one must ensure appropriate transducer levelling, timing [i.e., at end-diastole and end-expiration] and that the effect of intra-thoracic pressure is taken into account. These issues degrade the relationship between VExUS and Pra but also highlights a unique characteristic of venous Doppler. The pattern of venous Doppler reflects the pressure across the right atrial wall [i.e., the pressure within the right atrium relative to the pericardial pressure]. In other words, venous Doppler is not referenced to atmospheric pressure, like the Pra. In Brecher’s 1954 paper [8], he simulated decreased intrathoracic pressure [e.g., a spontaneous breath, which lowers measured Pra because transducers are referenced to atmosphere] by applying a suction cup to the right atrium and applying an outward force. The result was that the S wave shrank relative to the D wave, consistent with increased right atrial transmural pressure. The converse is also true, for example during pericardial tamponade, when measured Pra rises but transmural pressure falls because the pericardium rapidly fills with fluid. Clinically, the measured Pra might be 20 mmHg, but venous Doppler shows S wave > D wave morphology [21, 22] [Figure 2].
Figure 2: Transmural right atrial pressure versus measured right atrial pressure [Pra] during tamponade. OP1 is operating point 1 at baseline. The purple shaded bar shows the right atrial transmural pressure [i.e., Pra1 minus pericardial pressure, Ppc1] at baseline. With tamponade, operating point 2 [OP2] is formed because Ppc has increased to Ppc2. The result is that Pra increases significantly, but the Pra transmural pressure has decreased [purple shade between Ppc2 and Pra2].
What is VExUS?
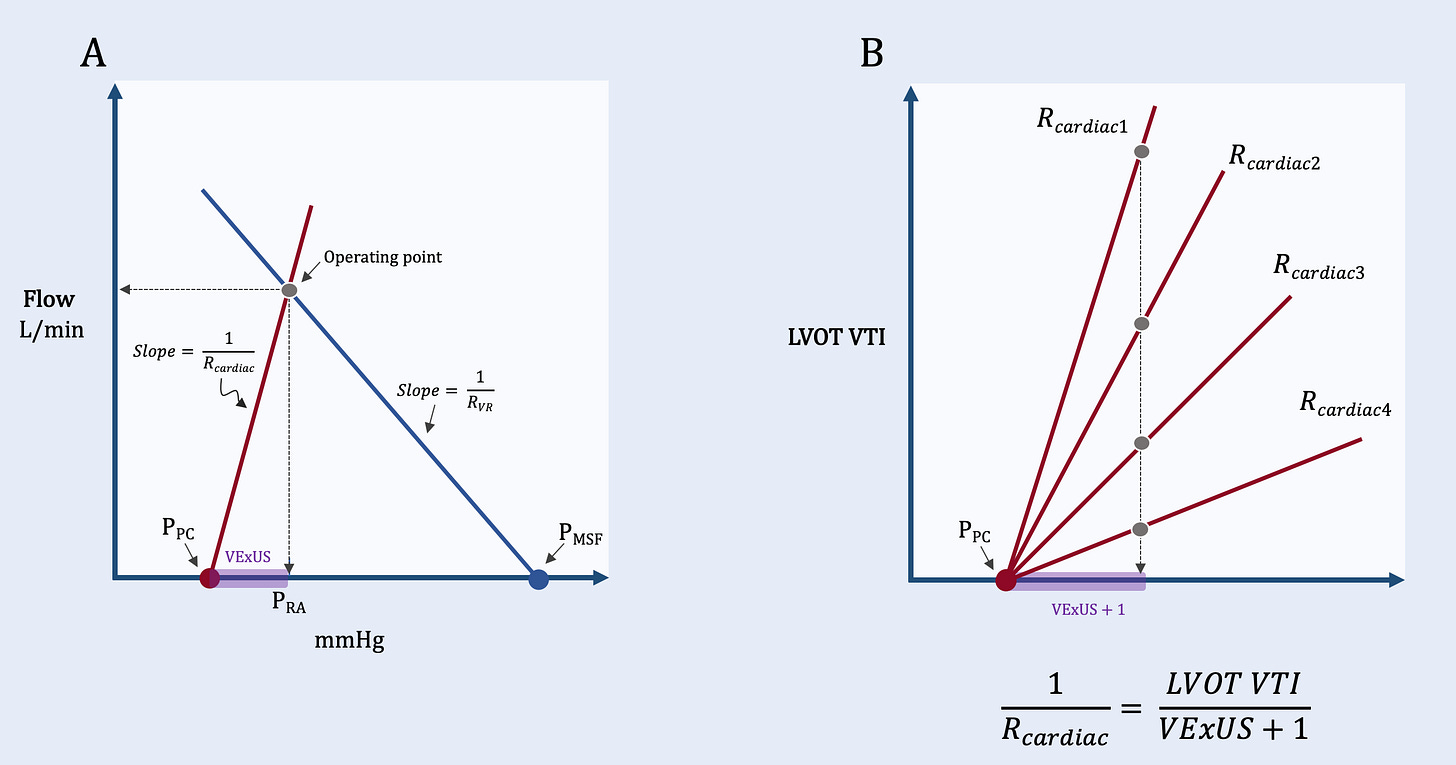
Given the above, VExUS – or venous Doppler more generally – is a measure of central venous wall stretch. A right atrium distended to its elastic limit diminishes the S wave relative to D wave, whereas a collapsed right atrium generates just the opposite. Thus, the uniqueness of venous Doppler is that it transduces the transmural Pra; in the context of a recently proposed ‘Geometrical model’ of the circulation [23], venous Doppler reflects the base of the ‘cardiac triangle’ [Figure 3].
Figure 3: VExUS and the Geometrical Model. A.) shows how VExUS integrates into the Geometrical model [23] as a surrogate for right atrial transmural pressure. B.) shows how the slope of the cardiac resistance (Rcardiac) curve can be approximated as the left ventricular outflow tract velocity time integral divided by VExUS plus 1. Rcardiac1 is the lowest cardiac resistance [i.e., best function] while Rcardiac4 is the highest cardiac resistance [i.e., worst function]. All 4 curves have the same VExUS plus 1 score, however, they have very different Rcardiac scores.
If the height of the cardiac triangle is approximated by the left ventricular outflow tract velocity time integral, then the slope of the cardiac function curve [Rcardiac] is solved as:
VExUS + 1 prevents zero in the denominator. Might this novel measure predict outcome in critically-ill patients [@ross_prager]?
Best,
JE
Please enjoy other posts in this series, now over a decade running!
Dr. Kenny is the cofounder and Chief Medical Officer of Flosonics Medical; he also the creator and author of a free hemodynamic curriculum at heart-lung.org. Download his free textbook here
References
1. Beaubien-Souligny W, Rola P, Haycock K, Bouchard J, Lamarche Y, Spiegel R, Denault AY, (2020) Quantifying systemic congestion with Point-Of-Care ultrasound: development of the venous excess ultrasound grading system. The ultrasound journal 12: 1-12
2. Shippy CR, Appel PL, Shoemaker WC, (1984) Reliability of clinical monitoring to assess blood volume in critically ill patients. Critical care medicine 12: 107-112
3. Parkin WG, Leaning MS, (2008) Therapeutic control of the circulation. J Clin Monit Comput 22: 391-400
4. Rothe CF, (1986) Physiology of venous return. An unappreciated boost to the heart. Arch Intern Med 146: 977-982
5. Rothe CF, (1993) Mean circulatory filling pressure: its meaning and measurement. J Appl Physiol (1985) 74: 499-509
6. Utrilla-Alvarez JD, Gopar-Nieto R, García-Cruz E, Lazcano-Díaz E, Jiménez-Rodrìguez GM, Rojas-Velasco G, Manzur-Sandoval D, (2023) Assessing the venous system: Correlation of mean systemic filling pressure with the venous excess ultrasound grading system in cardiac surgery. Echocardiography
7. Kenny J-ES, Moller PW, (2024) The venous excess ultrasound score (VExUS) and the mean systemic filling pressure. Echocardiography 41: e15727
8. Brecher GA, (1954) Cardiac Variations in Venous Return Studied With a New Bristle Flowmeter. American Journal of Physiology-Legacy Content 176: 423-430
9. Sivaciyan V, Ranganathan N, (1978) Transcutaneous doppler jugular venous flow velocity recording. Circulation 57: 930-939
10. Reynolds T, Appleton CP, (1991) Doppler flow velocity patterns of the superior vena cava, inferior vena cava, hepatic vein, coronary sinus, and atrial septal defect: a guide for the echocardiographer. Journal of the American Society of Echocardiography 4: 503-512
11. Abu-Yousef MM, Kakish M, Mufid M, (1996) Pulsatile venous Doppler flow in lower limbs: highly indicative of elevated right atrium pressure. AJR American journal of roentgenology 167: 977-980
12. Abu-Yousef MM, (1992) Normal and respiratory variations of the hepatic and portal venous duplex Doppler waveforms with simultaneous electrocardiographic correlation. Journal of ultrasound in medicine 11: 263-268
13. McNaughton DA, Abu-Yousef MM, (2011) Doppler US of the liver made simple. Radiographics 31: 161-188
14. Iida N, Seo Y, Sai S, Machino-Ohtsuka T, Yamamoto M, Ishizu T, Kawakami Y, Aonuma K, (2016) Clinical implications of intrarenal hemodynamic evaluation by Doppler ultrasonography in heart failure. JACC: Heart Failure 4: 674-682
15. Tang WW, Kitai T, (2016) Intrarenal venous flow: a window into the congestive kidney failure phenotype of heart failure? JACC: Heart Failure 4: 683-686
16. Argaiz ER, (2021) VExUS Nexus: Bedside Assessment of Venous Congestion. Adv Chronic Kidney Dis 28: 252-261
17. Longino A, Martin K, Leyba K, Siegel G, Gill E, Douglas IS, Burke J, (2023) Correlation between the VExUS score and right atrial pressure: a pilot prospective observational study. Crit Care 27: 205
18. Muñoz F, Born P, Bruna M, Ulloa R, González C, Philp V, Mondaca R, Blanco JP, Valenzuela ED, Retamal J, Miralles F, Wendel-Garcia PD, Ospina-Tascón GA, Castro R, Rola P, Bakker J, Hernández G, Kattan E, (2024) Coexistence of a fluid responsive state and venous congestion signals in critically ill patients: a multicenter observational proof-of-concept study. Crit Care 28: 52
19. Gupta K, Sondergaard S, Parkin G, Leaning M, Aneman A, (2015) Applying mean systemic filling pressure to assess the response to fluid boluses in cardiac post-surgical patients. Intensive Care Med 41: 265-272
20. Magder S, (2006) Central venous pressure monitoring. Current opinion in critical care 12: 219-227
21. Linden R, Byrd BF, (1987) Superior vena cava Doppler: a non-invasive method for the diagnosis of pericardial disease. International Journal of Cardiology 16: 145-153
22. Prager R, Pratte M, Kenny JE, Rola P, (2023) A Wireless, Wearable Carotid Doppler Ultrasound Aids Diagnosis and Monitoring of Pericardial Tamponade: A Case Report. Crit Care Explor 5: e0911
23. Kenny JS, (2023) A framework for heart-lung interaction and its application to prone position in the acute respiratory distress syndrome. Front Physiol 14: 1230654