ICU Physiology in 1000 Words: Airway Pressure Release Ventilation – Part 2
Jon-Emile S. Kenny MD [@heart_lung]
In the previous segment, time constants [t] – and their limitations – were described as a lesson in applied respiratory physiology for setting T-Low and prediction of auto-PEEP in airway pressure release ventilation [APRV]. In this brief missive an alternative approach to titrating T-low is described and critiqued. As well, P-High is considered with respect the sub-types of APRV breaths as a prelude to spontaneous ventilation, mechanical power and ergo-trauma described in the final chapter of this APRV trilogy.
Expiratory Flow Decay, Micro-strain & Micro-stress
Rather than calculate [t] – described in part 1 – one may simply use the change in expiratory flow as a surrogate of pressure decay. Thus, if expiratory flow falls by 50% one can expect that airway pressure diminishes by ~ 50% - assuming mono-exponential decline. Consequently, the clinician can titrate T-Low – and therefore auto-PEEP – by cycling to P-High when expiratory flow reaches some fraction of its peak value [see figure 1]. Indeed, in a number of fascinating animal studies [1-3], various expiratory flow-to-peak expiratory flow ratios [EF-PEFR] were studied in APRV for their effects upon alveolar recruitment and micro-strain.
Figure 1 – Expiratory flow curve on ventilator; note, this is simply figure 2 from part 1 inverted because expiratory flow is depicted this way on a ventilator. Lungs with short [t] in apple colour. Note how T-Low changes between short [t] lungs and normal t lungs when EF-PEFR of 75% is used as the cutoff to cycle to P-High. For reference, healthy, non-intubated patients reported [t] as 0.4 – 0.5 (s); ARDS [t] reported as 0.6 – 0.7 (s) [4]. Note the distinction between T-Low and [t]; the latter sets the slope of decay, the former is controlled by the clinician - set to when flow is 75% of its maximum value.
Each of the investigations of APRV found that when the EF-PEFR was 75% [i.e. when expiratory flow had fallen to 75% of its peak value] that alveoli – studied by unique microscopy techniques – showed the greatest expiratory area and the smallest amount of micro-strain. Micro-strain was defined as the change in volume from expiration to inspiration normalized to its expiratory value; as compared to low tidal volume ventilation [LTV] with high positive end-expiratory pressure [PEEP], micro-strain was similar but there was more absolute alveolar micro-recruitment in the EF-PEFR 75% APRV group. Additionally, the EF-PEFR of 75% was shown to redistribute gas volume from the respiratory bronchioles to the alveoli as compared to LTV [2]. For these reasons, it has been suggested that P-APRV maintain T-Low at a value resulting in an EF-PEFR of 75%.
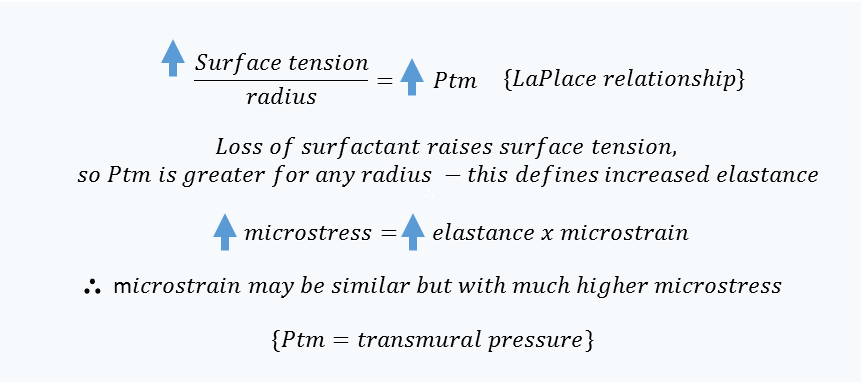
Notably, an EF-PEFR of 75% is expected to be much less than 1 [t] given the 25% fall in flow [recall that 1 [t] results in a 63% fall in the variable of interest]; a brief T-Low will recruit end-expiratory lung volume and generate auto-PEEP. Further, if end-expiratory volume is increased, then strain will, mathematically, fall [the denominator is larger]. Lastly, while micro-strain [inspiratory change in alveolar volume relative to expiratory volume] may fall, this does not necessarily speak to micro-stress. Stress is the pressure across the alveolus and is related to strain by elastance [or stiffness] of the structure being deformed [5]. In the aforementioned studies, surfactant wash-out models of ARDS were employed which is expected to greatly increase the surface tension of the alveolus – an increased micro-elastance – and therefore the pressure across it by LaPlace’s approximation [see figure 2].
Figure 2: theoretical relationship between microstrain [i.e. change in alveolar volume] and microstress [i.e. change in trans-mural alveolar pressure] at the level of the alveolus.
While the authors did not employ micro-transducers to measure alveolar pressure relative to interstitial/pleural pressure [i.e. measure micro-stress], a crude inference may be made by assessing the area of the non-airway, non-alveolar spaces visualized in their micrographs. This value was not reported, but can be deduced by subtracting the area of the conducting airways and alveoli from 100%. In other words, the fraction of space on microscopy that is not airway or alveolus reflects the summation of the interstitium, blood vessels and lymphatics. In the control group, this space made up 33% of the end-expiratory microscopic area while in the EF-PEFR of 75% group, it was only 27%. Thus, while end-expiratory alveolar area/recruitment was similar between the control and the EF-PEFR 75% groups, it came at the expense of ‘compressing’ the blood vessels, lymphatics and interstitium – by 6% [2]. While this may serve as a visual transduction of trans-alveolar pressure [i.e. ‘micro-stress’] it is, admittedly, speculative. Yet, the micro-recruitment observed in the EF-PEFR 75% group occurred at a meaningfully higher pressure-time product [double the LTV group] and tidal volume [double the LTV group] [1]. Thus, a reasonable supposition is that augmented micro-recruitment and diminished micro-strain amplified micro-stress; this critical point is reconsidered in the next installment – when discussing mechanical power and ‘ergotrauma.’
P-High, T-High and APRV Breath Types
Turning from the physiological considerations of T-Low, appreciation for the time spent at higher airway pressure [P-High] and spontaneous breaths are equally important when employing APRV in ARDS. As above, P-ARPV as defined by Habashi, requires more than 90% of the respiratory cycle time at P-High – to optimize alveolar recruitment. Typically T-High is around 5 seconds while P-High is close to the patient’s plateau pressure if switched from conventional ventilation.
How do the spontaneous breaths of APRV fit into the rotation between P-Low and P-High? Different breaths types in APRV have previously been defined and form the basis for analysis of ergotrauma below [6, 7]:
Figure 3: Breath types during APRV - A Breath: spontaneous at P-Low; B Breath: spontaneous at P-High; C Breath: spontaneous at the initiation of P-High; D Breath: ventilator breath from P-Low to P-High; E Breath; spontaneous breath initiated at transition from P-High to P-Low. Note that only the D breath is purely from the ventilator
As can be seen, the types of breath present will vary as a function of the duration of T-High relative to T-Low. Indeed, shortening T-Low essentially abolishes all breaths except B and D types [8]. As T-High is increased relative to T-Low, ventilation and work is transferred from the ventilator to the patient [8]. As P-ARPV demands T-Low values less than 1 [t] in duration, presumably all breaths are either B type [i.e. spontaneously during P-High] or D type [i.e. ventilator controlled P-Low to P-High].
Given the description of breath types in P-APRV above, the following and final post of this ‘APRV trilogy’ will turn to mechanical power applied to the lung skeleton during P-APRV and how pulmonary versus extra-pulmonary ARDS will greatly affect the degree of strain and stress felt by the lung.
Please check out other posts in the ‘1000 Word’ series,
JE
Dr. Kenny is the cofounder and Chief Medical Officer of Flosonics Medical; he is also the creator and author of a free hemodynamic curriculum at heart-lung.org
References
Kollisch-Singule, M., et al., Mechanical breath profile of airway pressure release ventilation: the effect on alveolar recruitment and microstrain in acute lung injury. JAMA surgery, 2014. 149(11): p. 1138-1145.
Kollisch-Singule, M., et al., Airway pressure release ventilation reduces conducting airway micro-strain in lung injury. Journal of the American College of Surgeons, 2014. 219(5): p. 968-976.
Kollisch-Singule, M., et al., Effect of airway pressure release ventilation on dynamic alveolar heterogeneity. JAMA surgery, 2016. 151(1): p. 64-72.
Al-Rawas, N., et al., Expiratory time constant for determinations of plateau pressure, respiratory system compliance, and total resistance. Critical Care, 2013. 17(1): p. R23.
Gattinoni, L., et al., The" baby lung" became an adult. Intensive care medicine, 2016. 42(5): p. 663-673.
Kallet, R.H., Patient-ventilator interaction during acute lung injury, and the role of spontaneous breathing: part 2: airway pressure release ventilation. Respiratory care, 2011. 56(2): p. 190-206.
Calzia, E., et al., Pressure-time product and work of breathing during biphasic continuous positive airway pressure and assisted spontaneous breathing. American journal of respiratory and critical care medicine, 1994. 150(4): p. 904-910.
Neumann, P., et al., Influence of different release times on spontaneous breathing pattern during airway pressure release ventilation. Intensive care medicine, 2002. 28(12): p. 1742-1749.