ICU Physiology in 1000 Words: Venous Doppler & Veno-Cardiac Coupling – Part 1
Jon-Emile S. Kenny MD [@heart_lung]
Background
Concepts have been clanging around my head since I participated in Philippe Rola’s [@thinkingCC] sedulous Hospitalist & Resuscitationist Conference in Montreal. Initially, the abstractions of ventriculo-arterial coupling, Guytonian physiology and venous Doppler seemed insuperably sundered; but the cognitive haranguing recently gave way to harmony.
What is ventriculo-arterial coupling and how does it relate to Guyton’s formulation of the circulatory system? How might venous Doppler of the great veins fit into these frameworks and could the un-coupling of venous-from-cardiac power act as an underlying explanatory theory for aberrant venous Doppler morphology?
The Arterial Load: Ea
The arterial load – typically termed ‘afterload’ – is a complex matter; it is most certainly not simply the calculated ‘systemic vascular resistance [SVR].’ SVR is derived from a simplistic model of the circulatory system – one that does not account for other elements that determine true arterial load. For instance, the vasculature carries both a compliance – describing its distensibility – as well as a ‘characteristic impedance’ which, along with ‘classical resistance’ [i.e. SVR] make up the ‘3-element Windkessel’ model of arterial load.
Yet the challenge with the 3-element model is measurement at the bedside. Fortunately, groundbreaking work by Sunagawa et al. over 30 years ago revealed that the slope of the left-ventricular end-systolic pressure [ESP] relative to the stroke volume [SV] was an adequate surrogate for total arterial load [1-3]; accordingly, the value of the ESP divided by the SV is the arterial elastance [Ea]. The Ea is a ‘lumped’ index that reflects all elements of the Windkessel model. Ea has units of mmHg per mL because it describes the change in central aortic pressure relative to a change in blood volume. Ea is represented by the purple line in figure 1; a normal value is 2.2 +/- 0.8 mmHg/mL [4]. An increase in arterial elastance [e.g. hypertensive emergency] shifts the Ea curve up-and-rightwards [see figure 1B] while a decrease in Ea [e.g. vasodilation] shifts the Ea curve down-and-leftwards [5, 6].
Figure 1: A - represents normal coupling between the arterial [Ea-purple] load and ventricular [ESPVR-Ees - dark blue] function. SV is stroke volume, ESV is end-systolic volume, EDV is end-diastolic volume, EDPVR is end-diastolic pressure volume relationship. Stroke work is the blue shaded area [circled 1] while potential work is the red-shaded area [circled 2]. B reveals an elevated Ea/Ees or ventriculo-arterial un-coupling.
The Left Ventricular Function: Ees
By similar reasoning to the Ea, the left ventricular end-systolic elastance [Ees] is a measure that describes the ‘stiffness’ of the left ventricle at end-systole. The Ees is therefore a ‘lumped’ index of left ventricular performance comprising contractility as well as geometric and other structural properties of the LV [2, 7]. Importantly, however, acute changes in Ees reflect acute changes in LV contractility that are load-independent; that is, unaffected by contemporaneous changes in preload and afterload. The LV Ees is also indicated in figure 1 and a normal value is essentially identical to the Ea, that is, 2.3 +/- 1.0 mmHg/mL [4]. An increase in contractility [e.g. cardiac beta-2 receptor stimulation] shifts the Ees curve up-and-leftwards, while a decrease in contractility [e.g. septic cardiomyopathy] shifts it down-and-rightwards [see figure 1B] [4-6].
Ventriculo-Arterial Coupling & Cardiac Efficiency
Given that Ea and Ees have identical units and nearly equal values, their relationship – the Ea/Ees ratio – is normally 1.0 +/- 0.36 [2]. A normal Ea/Ees ratio is visualized in figure 1A while an abnormal [i.e. increased or uncoupled] ratio is seen in figure 1B. Note that an increase in Ea, a decrease in Ees, [or both – seen in figure 1B] will increase the Ea/Ees ratio and, consequently, the end-systolic volume [compare ESVa to ESVb in figure 1A and 1B]. Increased end-systolic volume will decrease stroke volume [SV], ceteris paribus.
What is also evident from figure 1B is that impaired coupling between the ventricle and the arteries [i.e. an increased Ea/Ees ratio] diminishes cardiac efficiency [8]. The efficiency of the heart can be visualized as the ratio between the work required for the stroke volume [i.e. stroke work – blue shade in figure 1] relative to the total work for the contraction. The total work for a cardiac contraction is equal to the potential work [in red shade – marked by the circled 2] plus the stroke work [in blue shade – marked by the circled 1]. Thus, the less-efficient heart leaves excess potential work behind during cardiac contraction; this concept will be revisited later under the guise of ‘veno-cardiac coupling.’
How is any of the above clinically-relevant? A recent and fascinating paper evaluated the effect of volume expansion as well as norepinephrine and dobutamine infusions upon VAC in patients with un-resuscitated septic shock [8]. The authors used the oft-cited, single-beat method described by Chen et al. to non-invasively measure the Ees [9]. As outlined earlier, the authors observed that early volume-expansion with 30 mL/kg acutely-improved Ees and cardiac efficiency. How might intravenous fluids improve an index of cardiac contractility? It may have been secondary to increased coronary perfusion and, accordingly, myocardial oxygen delivery. It is also possible that a relatively cold [i.e. room temperature] crystalloid infusion increased adrenergic tone. Lastly, despite being validated invasively on 43 patients, the single-beat method of Chen et al. may be inapplicable to septic patients and/or sensitive to the afterload-reducing effects of intravenous fluids [10-12].
Ventriculo-arterial Coupling & Guytonian Physiology
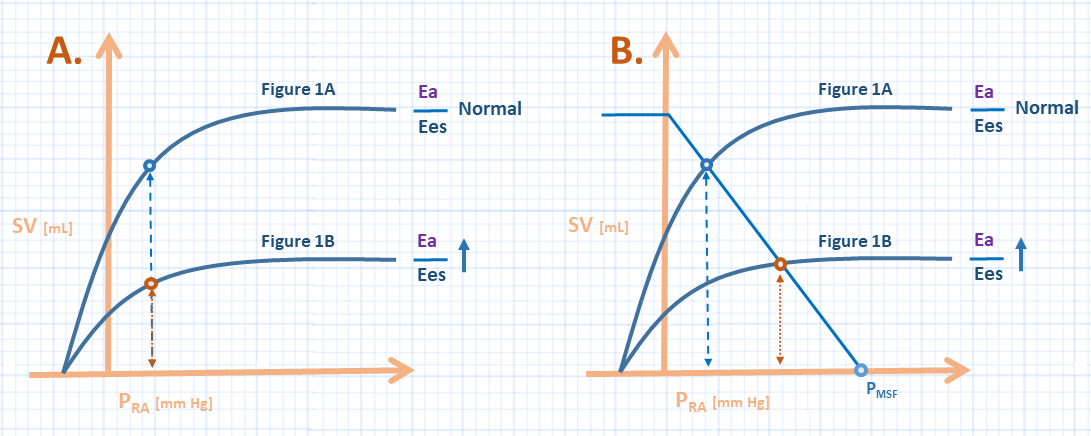
But how does the uncoupling of the ventricle(s) from their arterial tributaries relate to Guyton’s framework of the cardiovascular system? Figure 2A communicates this relationship [6]. If the y-axis becomes stroke volume – rather than cardiac output – one observes that the uncoupled ventricle has a smaller SV for a given filling pressure. Accordingly, whether the uncoupling is right-sided, left-sided, or both – the outcome is that the ‘lumped’ Frank-Starling curve is shifted down-and-rightwards.
Figure 2: A represents the effect of increasing the Ea/Ees ratio on the Guyton diagram; the y-axis is stroke volume [rather than cardiac output - SV x HR] and x-axis is right atrial pressure. These curves correspond to figure 1A and 1B [see above]. B represents the addition of mean systemic filling pressure [Pmsf] and venous return to the heart with ventriculo-arterial decoupling. The light blue curve represents the venous return function.
Yet if we focus only on how the cardiac pump interacts with the arterial tree, we ignore half of the cardiovascular picture; the venous return also cooperates with the pump! In other words, there is an energy stored within the venous vasculature that is transferred to the pump function – which then interacts with the arteries as per above. Figure 2B demonstrates how the venous return must also be considered. A patient with a certain volume status and stressed venous volume – as measured by the mean systemic filling pressure – who then suffers ventriculo-arterial uncoupling would see his or her right atrial pressure rise as the impaired pump function accepts venous blood return. As elaborated in Part 2, the patient with impaired VAC exhibits impaired cardiac performance [13] because the right atrial filling pressure rises relative to the mean systemic filling pressure – easily visualized in figure 2B.
Find Part 2 here.
Celebrating 5 years of the ‘1000 Word’ series!
JE
Dr. Kenny is the cofounder and Chief Medical Officer of Flosonics Medical; he is also the creator and author of a free hemodynamic curriculum at heart-lung.org
References
Sunagawa, K., et al., Left ventricular interaction with arterial load studied in isolated canine ventricle. American Journal of Physiology-Heart and Circulatory Physiology, 1983. 245(5): p. H773-H780.
Chantler, P.D., E.G. Lakatta, and S.S. Najjar, Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. Journal of applied physiology, 2008. 105(4): p. 1342-1351.
Kelly, R.P., et al., Effective arterial elastance as index of arterial vascular load in humans. Circulation, 1992. 86(2): p. 513-521.
Guarracino, F., et al., Ventriculoarterial decoupling in human septic shock. Critical Care, 2014. 18(2): p. R80.
Guarracino, F., R. Baldassarri, and M.R. Pinsky, Ventriculo-arterial decoupling in acutely altered hemodynamic states. Critical Care, 2013. 17(2): p. 213.
Walley, K.R., Left ventricular function: time-varying elastance and left ventricular aortic coupling. Critical care, 2016. 20(1): p. 270.
Borlaug, B.A. and D.A. Kass, Ventricular–vascular interaction in heart failure. Cardiology clinics, 2011. 29(3): p. 447-459.
Guarracino, F., P. Bertini, and M.R. Pinsky, Cardiovascular determinants of resuscitation from sepsis and septic shock. Critical Care, 2019. 23(1): p. 118.
Chen, C.-H., et al., Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. Journal of the American College of Cardiology, 2001. 38(7): p. 2028-2034.
Yotti, R., et al., Validation of noninvasive indices of global systolic function in patients with normal and abnormal loading conditions: a simultaneous echocardiography pressure–volume catheterization study. Circulation: Cardiovascular Imaging, 2014. 7(1): p. 164-172.
Kumar, A., et al., Preload-independent mechanisms contribute to increased stroke volume following large volume saline infusion in normal volunteers: a prospective interventional study. Critical Care, 2004. 8(3): p. R128.
García, M.I.M., et al., Effects of fluid administration on arterial load in septic shock patients. Intensive care medicine, 2015. 41(7): p. 1247-1255.
Parkin, W.G. and M.S. Leaning, Therapeutic control of the circulation. Journal of clinical monitoring and computing, 2008. 22(6): p. 391-400.