ICU Physiology in 1000 Words: Volutrauma or Barotrauma?
Jon-Emile S. Kenny MD [@heart_lung]
In a recent and excellent open-access review, Gattinoni, Quintel and Marini ask which is worse, volutrauma or atelectrauma [1]? This concise review is an absolute must-read and forms the fabric from which this short article assembles. Last spring – in Montreal – I was asked a few questions about volutrauma and its relationship to barotrauma. I hope to clarify these two processes by sewing together basic physiological principles and clinically-relevant values into a pleasing patchwork quilt of ICU physiology.
Volume and Strain
The absolute volume applied to the lung is an important determinant of biological injury. However, equally-important is the absolute volume delivered relative to the baseline volume of the lung [i.e. functional residual capacity or FRC]. The latter is often referred to as the ‘baby lung’ in ARDS because FRC can be quite variable and quite small [2, 3]; FRC in ARDS is typically unknown. Imagine, for example, two different patients – one with an FRC of 1500 mL and the other with an FRC of only 100 mL because of severe lung injury [i.e. the ‘baby lung’]. Delivery of 500 mL to the first patient results in a lung strain of 0.33. Lung strain is the tidal volume divided by the FRC [i.e. 500 mL divided by 1500 mL]. However, in the second patient, the same tidal volume results in a lung strain of 5.0 [i.e. 500 mL divided by 100 mL]! These two extreme examples illustrate why there is no one-size-fits-all tidal volume for all patients with ARDS. If the volume of the baby lung is unknown, true lung strain is also unknown but it is clear that keeping tidal volume small will also keep lung strain small.
Pressure and Stress
The pressure across a distensible material is known as stress and is directly related to strain [determined as above] [4, 5] - see equation 1.
Equation 1: stress and strain are directly related by a constant - K - the specific elastance
Pressure is often measured clinically as the plateau pressure on the ventilator. Analogous to volume, it is not solely the value of pressure in the airway that is important, but rather the pressure in the airway relative to the pressure around the lung – the pleural pressure. When considering the stress or pressure across the lung [plateau relative to pleural pressure] one can anticipate clinical scenarios whereby both plateau and pleural pressure rise in tandem such that the plateau pressure is very high, but because the pleural pressure is also high, the pressure [i.e. stress] across the lung is actually normal. An example in the ICU may be morbid obesity where the weight placed upon the chest wall results in an increase in both pleural pressure and plateau pressure.
A more physiologically-exciting example is that of fighter-pilots wearing anti-gravity suits. As a defense against gravity-induced loss-of-consciousness [GLOC], anti-gravity suits pressurize the thorax as this helps maintain a pressure gradient for blood flow from the left ventricle to the brain. One mechanism of these suits is the application of an airway pressure upwards of 80 – 90 cm H2O! If one considers only the airway pressure this will lead one to speciously infer a very high lung stress. However, the chest wall stiffens during high-gravity maneuvers; in addition, an inflatable bladder around the abdomen and chest – in some suits – also braces the chest wall. As a result, the pleural pressure also increases dramatically such that the very high airway pressure is tempered by an augmented pleural pressure – lung stress is actually normal!
Volutrauma versus Barotrauma
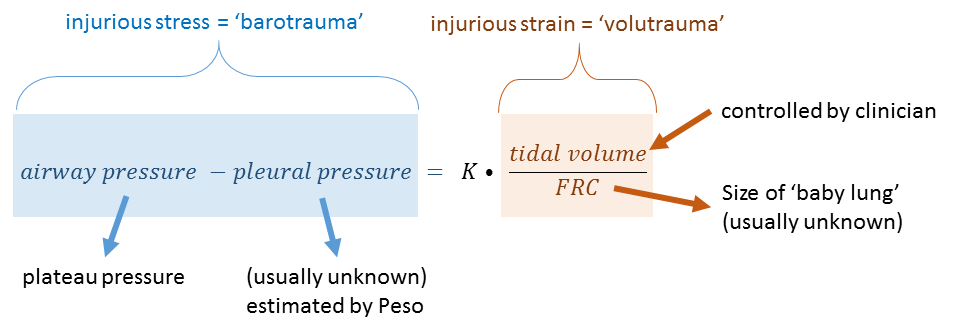
So which is the mechanism of lung injury, volutrauma or barotrauma? They are – in essence – the same thing but only when one considers tidal volume in relation to FRC [i.e. strain] and airway pressure in relation to pleural pressure [i.e. stress] [4]. Equation 1 above is fleshed out into a clinically-relevant relationship in equation 2.
Equation 2: Colour-coding here matches equation 1. The stress across the lung in blue [which determines barotrauma] is directly related to lung strain in orange [which determines volutrauma]; they are related by the specific elastance - K. Note that barotrauma and volutrauma are mathematically equal when one considers the airway pressure relative to the pleural pressure [estimated by the esophageal pressure - Peso], the tidal volume relative to the functional residual capacity - FRC - and the specific elastance. Stress raisers - see text - amplify the left side of the equation. This equation assumes volume control ventilation.
One can see that stress and strain are directly related by a constant – known as the ‘specific elastance’ [K]. Elastance is the inverse of compliance – a measure of the ‘stiffness’ of the material. In humans, this value is about 12 cm H2O. In other words, increasing the tidal volume of the lung relative to the FRC by a factor of 1.0 [i.e. a strain of 1.0] will result in a trans-pulmonary pressure of 12 cm H2O. Total lung capacity occurs at a lung strain of about 2.5 – which produces in a trans-pulmonary pressure of about 30 cm of H2O in humans. Interestingly, at least some lung units see their collagen fibers completely distended at a trans-pulmonary pressure as low as 21 cm H2O [6] which corresponds to a lung strain of roughly 1.5 to 2.0 – a value also known to provoke lung injury in swine [7].
What may seem paradoxical is that the specific elastance remains unchanged even in severe ARDS [2, 8]; in other words the numerical relationship between strain and stress stays constant as the functional lung size shrinks in ARDS. Accordingly, strain and stress rise as FRC shrinks in ARDS rather than as a consequence of increased in elastance [decreased compliance].
Atelectasis and Stress Raisers
The mechanism by which atelectasis drives lung injury may be secondary to excessive mechanical stress upon the lung skeleton [4, 5]. A ‘stress raiser’ [or riser] occurs when materials of differing elasticity are located adjacent to one another [9]. In this situation, the less compliant area augments radial stress at the interface of the two units upon deformation. An atelectatic unit acts as a stress raiser on surrounding lung parenchyma. In a classic – but theoretical – paper by Mead and colleagues, a stress raiser was found to augment local stress by a factor of 4.64 [9]. Thus, a trans-pulmonary pressure of 21 cm H2O at a stress raiser would actually be 21 cm H2O x 4.64 or 97 cm H2O – certainly injurious! Nevertheless, empirical evidence has found this multiplication factor to be closer to 2.0 [10]. If we were to factor the stress raiser into the equation above, we can see the result is – in essence – a functional increase in lung elastance [decrease in compliance] [1]. Given this factor of 2.0, some have suggested keeping the trans-pulmonary driving pressure less than 12 cm H2O [11]. Importantly, stress raisers are diminished by both positive end-expiratory pressure [PEEP] and prone position – the latter does not, however, increase mechanical power [12].
Best,
JE
Dr. Kenny is the cofounder and Chief Medical Officer of Flosonics Medical; he is also the creator and author of a free hemodynamic curriculum at heart-lung.org
References
Gattinoni, L., M. Quintel, and J.J. Marini, Volutrauma and atelectrauma: which is worse?, 2018, BioMed Central.
Gattinoni, L. and A. Pesenti, The concept of “baby lung”. Intensive Care Med, 2005. 31(6): p. 776-784.
Gattinoni, L., et al., The" baby lung" became an adult. Intensive care medicine, 2016. 42(5): p. 663-673.
Tonetti, T., et al., Driving pressure and mechanical power: new targets for VILI prevention. Annals of translational medicine, 2017. 5(14).
Gattinoni, L., et al., The future of mechanical ventilation: lessons from the present and the past. Critical Care, 2017. 21(1): p. 183.
Protti, A., et al., Lung anatomy, energy load, and ventilator-induced lung injury. Intensive care medicine experimental, 2015. 3(1): p. 34.
Protti, A., et al., Lung stress and strain during mechanical ventilation: any safe threshold? Am J Respir Crit Care Med, 2011. 183(10): p. 1354-1362.
Gattinoni, L., et al., Relationships between lung computed tomographic density, gas exchange, and PEEP in acute respiratory failure. Anesthesiology, 1988. 69(6): p. 824-832.
Mead, J., T. Takishima, and D. Leith, Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol, 1970. 28(5): p. 596-608.
Cressoni, M., et al., Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med, 2014. 189(2): p. 149-158.
Kassis, E.B., S. Loring, and D. Talmor, Esophageal pressure: research or clinical tool? Medizinische Klinik-Intensivmedizin und Notfallmedizin, 2018: p. 1-8.
Gattinoni, L. and M. Quintel, How ARDS should be treated. Critical Care, 2016. 20(1): p. 1.