ICU Physiology in 1000 Words: The Driving Power & Ventilator-Induced Lung Injury
Jon-Emile S. Kenny MD [@heart_lung]
The mechanical power applied to the lung is a risk factor for ventilator or ventilation-induced lung injury [VILI] [1-4]. But can the work done to the lung over time be homogenized into a single value? Could different components of the power equation carry different VILI risk beyond their mathematical inequalities [2]?
One possible avenue to explore these questions is by studying positive end-expiratory pressure [PEEP] which should linearly increase mechanical power and, therefore, VILI risk [5]. But others criticize this reasoning – noting that PEEP should have a ‘U-shaped’ effect [6, 7]. In other words, increasing PEEP should linearly increase mechanical power, but not necessarily VILI.
A very recent porcine investigation provides some answers to the questions above [4]; but before explicating these results, a primer on the effect of PEEP on power is justified.
PEEP & the Viscoelastic Lung
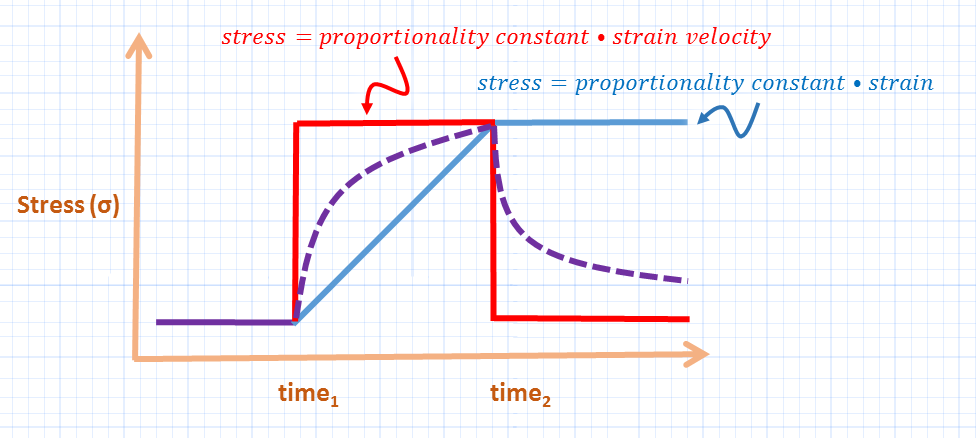
The degree of stress developed in a viscous material [think putty], is quite different from a purely elastic material [think rubber band] when submitted to a given strain [see figure 1] [8]. Recall that strain is an increase in length – or volume – relative to baseline [9, 10]. In a viscous material, the stress felt is proportional to strain velocity, while in an elastic material the stress is proportional to the strain and the material’s inherent ‘stiffness’ or specific elastance.
In a purely elastic material, the energy required to stress the object is stored in potential energy which is recaptured upon strain release; in a viscous material, this energy is lost as heat and rearrangement of the underlying material structure.
The lung is viscoelastic – its stress response to a given strain is somewhere between a purely viscous and elastic material [8, 11]. Accordingly, the absolute strain and the strain velocity mediate stress and, therefore, lung injury. How does PEEP affect absolute strain and strain velocity?
Figure 1: The viscoelastic lung [dotted purple] has stress properties in between those of purely elastic material [light blue line, think rubber band] and purely viscous material [red line, think putty]. Thus the stress of the lung depends upon both intrinsic properties and the rate of expansion [see text]. Stress is on y-axis, duration of applied strain on x-axis.
Firstly, when total strain remains constant [i.e. constant lung volume change], but this total strain is composed of increasing levels of PEEP [e.g. 100% tidal strain versus 75% tidal strain and 25% PEEP strain], there is less VILI when there is a greater PEEP fraction [12] – when all other variables like respiratory rate and I:E ratio are constant. Presumably, because a smaller tidal strain means a smaller volume change per unit time [i.e. strain rate], there is less viscous-mediated stress across the lung and less lung injury [8].
Secondly, PEEP ‘homogenizes’ the lung [as does pronation]; more simply, PEEP reduces the number of ‘stress raisers [13].’ Stress raisers act as local stress amplifiers when adjacent materials of differing elastance receive the same strain. Mathematically, stress raisers augment the local stress by 4.5 [14] though this is probably closer to a doubling clinically [5, 15].
In summary, PEEP reduces strain rate which reduces viscous stress and PEEP homogenizes the lung which reduces local specific elastance or elastic stress.
PEEP & the Mechanical Power
A very recent porcine study investigated the effect of 6 levels of PEEP with tidal volume, flow and respiratory rate all held constant [4]. One group received zero end-expiratory pressure [ZEEP] while the other 5 groups entertained progressively higher PEEP. Amongst the 5 groups that received PEEP, there was a linear rise in mechanical power and risk of VILI. Importantly, however, the ZEEP group had equivalent mechanical power to the low PEEP groups, but had lung injury comparable to the high PEEP groups. Thus, while overall mechanical power is an important determinant of VILI, it is not absolute, because the ZEEP group had relatively low power. A hypothetical illustration of ZEEP versus PEEP and mechanical power is seen in figure 2.
Moreover, the fraction of power dedicated to tidal ventilation [i.e. ‘driving power’] seemingly better correlated with VILI. In the ZEEP group at baseline, nearly 60% of the total power per breath was due to the elastic, tidal volume component, while in the low PEEP groups, this fraction fell to about 40%. The fall in driving power probably resulted from improved pulmonary compliance from atelectatic lung recruitment – decreasing driving pressure [see figure 2]. Considering above, PEEP improved the elastic stress – given that the strain rate was constant – which reduced local stress raisers and mitigated VILI.
Figure 2: Hypothetical effect of PEEP on mechanical power sub-components [power is work over time]. The bottom graphic represents the total inspiratory lung work for a single breath [driving work in red, resistive work in blue] under ZEEP conditions. The lung elastance at ZEEP [ELzeep] is high, or stiff. Note that the total work at ZEEP [total area] is very similar to the total work of the top graph which represents the application of PEEP. While total work [or power if respiratory rate equal] is the same, the ZEEP condition is much more damaging, perhaps because of the high 'driving power' [driving work in red, multiplied by respiratory rate]. Note that PEEP decreases lung elastance [ELpeep - dotted blue line], decreases driving work and resistive work [shaded blue]. Volume on y-axis, pressure on x-axis. Note the difference between the driving pressure [Pplat or plateau pressure minus the PEEP - both represented by targets]; ΔV is tidal volume.
How to Operationalize the Driving Power?
Given the above, we are led back to Marini’s prescient description of the ‘driving power [7].’ If this is the most important sub-component of the mechanical power, how could one go about operationalizing this at the bedside?
Firstly, concentrating on the three key determinants of the driving power is sensible [i.e. tidal volume, respiratory rate and lung elastance]. Previously, Gattinoni’s group found that tidal volume augmented total power exponentially by a factor of 2.0, while respiratory rate increased power exponentially by a factor of 1.4 [2]. Importantly, reducing both respiratory rate and tidal volume decrease all of the other power sub-components [e.g. resistive, elastic-PEEP and elastic-tidal volume - see figure 2]. Ideally, tidal volume is reduced with knowledge of the volume of the ‘baby lung’ - to minimize strain [10].
The next component of the driving power – the lung elastance – can be reduced by increasing PEEP. Titrating the effect of PEEP on lung elastance may be executed by either the stress index or driving pressure – as described previously. Thus, increasing PEEP-related mechanical power seems prudent when offset by a decrease in the fraction of driving power. If PEEP fails to decrease pulmonary elastance – and therefore driving power – then prone position should be considered [13, 16].
Once driving power is optimized, then the resistive work is minimized. Importantly, PEEP decreases airway resistance by increased lung volume. As well, reduced I:E ratio should also reduce VILI risk as flow, like tidal volume, augments mechanical power exponentially by a factor of 2.0 [2].
Please check out other articles in this series,
JE
Dr. Kenny is the cofounder and Chief Medical Officer of Flosonics Medical; he is also the creator and author of a free hemodynamic curriculum at heart-lung.org
References
Cressoni, M., et al., Mechanical power and development of ventilator-induced lung injury. Anesthesiology: The Journal of the American Society of Anesthesiologists, 2016. 124(5): p. 1100-1108.
Gattinoni, L., et al., Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med, 2016. 42(10): p. 1567-1575.
Tonetti, T., et al., Driving pressure and mechanical power: new targets for VILI prevention. Annals of translational medicine, 2017. 5(14).
Collino, F., et al., Positive end-expiratory pressure and mechanical power. Anesthesiology: The Journal of the American Society of Anesthesiologists, 2019. 130(1): p. 119-130.
Gattinoni, L., et al., The future of mechanical ventilation: lessons from the present and the past. Critical Care, 2017. 21(1): p. 183.
Huhle, R., et al., Is mechanical power the final word on ventilator-induced lung injury?—no. Annals of translational medicine, 2018. 6(19).
Marini, J.J. and S. Jaber, Dynamic predictors of VILI risk: beyond the driving pressure, 2016, Springer.
Protti, A., E. Votta, and L. Gattinoni, Which is the most important strain in the pathogenesis of ventilator-induced lung injury: dynamic or static? Curr Opin Crit Care, 2014. 20(1): p. 33-38.
Gattinoni, L. and A. Pesenti, The concept of “baby lung”. Intensive care medicine, 2005. 31(6): p. 776-784.
Gattinoni, L., et al., The" baby lung" became an adult. Intensive care medicine, 2016. 42(5): p. 663-673.
Marini, J.J., Dissipation of energy during the respiratory cycle: conditional importance of ergotrauma to structural lung damage. Current opinion in critical care, 2018. 24(1): p. 16-22.
Protti, A., et al., Lung stress and strain during mechanical ventilation: any difference between statics and dynamics? Crit Care Med, 2013. 41(4): p. 1046-1055.
Gattinoni, L. and M. Quintel, How ARDS should be treated. Critical Care, 2016. 20(1): p. 1.
Mead, J., T. Takishima, and D. Leith, Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol, 1970. 28(5): p. 596-608.
Cressoni, M., et al., Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med, 2014. 189(2): p. 149-158.
Vieillard-Baron, A., et al., Experts’ opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med, 2016. 42(5): p. 739-749.