ICU Physiology in 1000 Words: Cardiothoracic Uncoupling & Venous Ergotrauma
Jon-Emile S. Kenny MD [@heart_lung]
Pulmonary Elastic Ergotrauma
There are hidden hemodynamics in respiratory mechanics, as previously discussed. Recall that these relationships are illustrated by comparing volume-pressure, or compliance curves. Trans-pulmonary pressure, which approximates pulmonary stress, is the pressure in the airway compartment less the pleural pressure [1]. In a passive, mechanically-ventilated patient, the trans-pulmonary pressure is the lateral distance between the respiratory system compliance curve [which approximates alveolar pressure] and the chest wall compliance curve [which approximates pleural pressure] [figure 1].
When a breath is delivered, pressure rises to the plateau pressure, after an inspiratory hold. But what does the area between the respiratory system compliance curve and chest wall compliance curve represent? The area bounded by the pressure-volume curves depicts work, or energy [e.g. in joules]. As initially proposed by Cressoni and colleagues [2, 3], excessive energy applied to the lung over time [i.e. power, in watts] is a unifying explanatory model for ventilation-induced lung injury. While total energy per breath includes both resistive components [e.g. flow, resistance] and elastic components [e.g. volume, compliance] this discussion will ignore the former, for simplicity [figure 1].
Figure 1: Illustration of work across the lung. A. shows normal conditions. Pleural pressure [Ppl] is estimated by esophageal pressure [Peso] and tracks the passive chest wall compliance curve [Ccw]. The breath starts at a positive end-expiratory pressure [PEEP] of 5 cm H2O; the tidal volume [red arrow y-axis] is about 450 mL. The breath ends at the plateau pressure [Pplat] on the respiratory system compliance curve [Crs]. The distance between the Crs and Ccw illustrates the trans-pulmonary pressure [Ptp] which is the stress across the lung from the airway pressure [Paw] to the Ppl. The shaded pink area is elastic PEEP work and the shaded red area is elastic driving work. B. shows pulmonary ARDS. The Crs is shifted down and Ccw is assumed to be constant and normal. PEEP and tidal volume are the same as A. The driving pressure [∆P] rises and elastic work, or power over time, rises significantly. This is ‘alveolo-pleural uncoupling.’
The energy applied to the lung over time must also affect the pulmonary vasculature [4]. Thus, ergotrauma is also a hemodynamic event. One perspective might be that energetic ‘uncoupling’ between the airway and pleural compartments disturbs the pulmonary vasculature by augmenting pulmonary arterial impedance.
Ventriculo-arterial Uncoupling
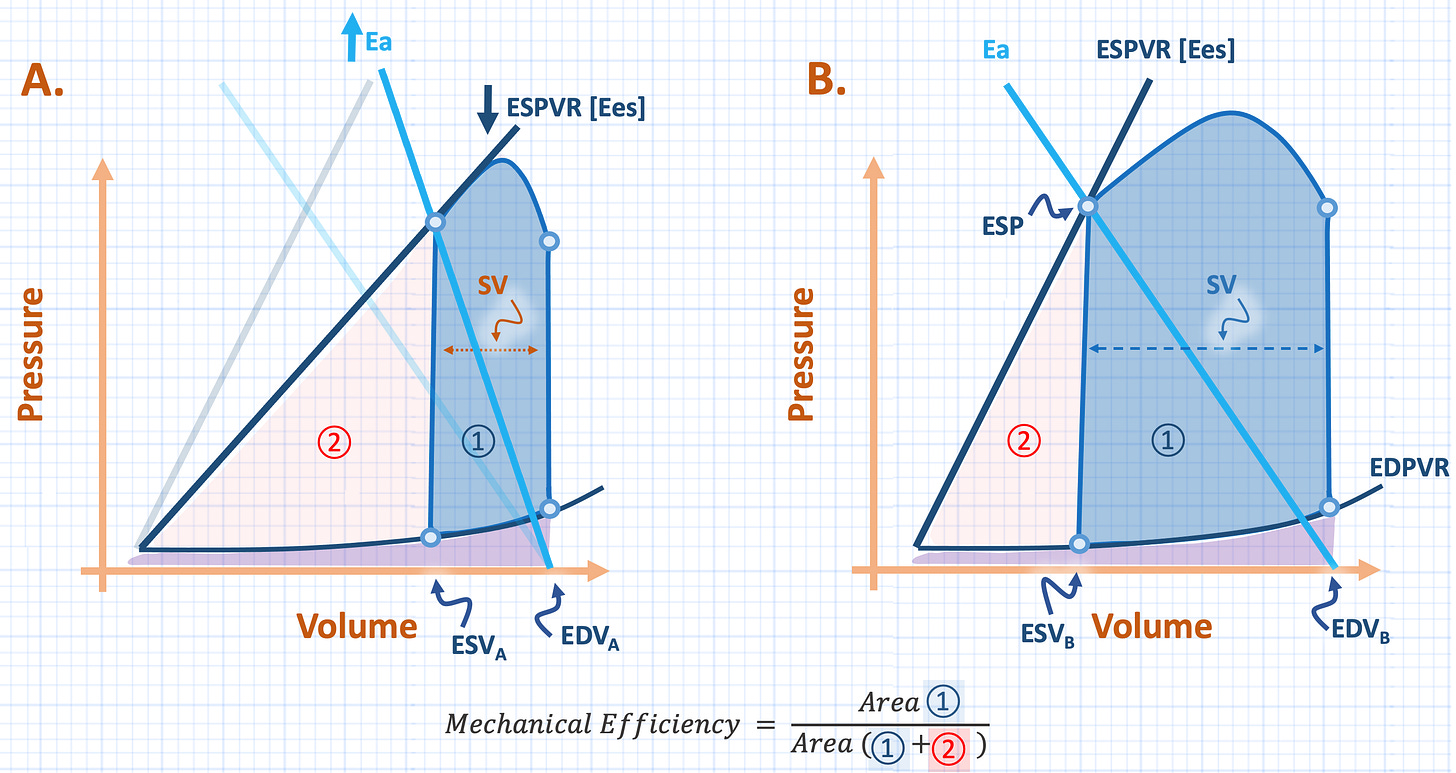
But pressure-volume curves, and the area between them, are also discussed in cardiovascular physiology. For example, on the Sagawa pressure-volume relationship [5], the area bound between the end-diastolic and systolic pressure-volume relationships represents myocardial work. Further, this work is partitioned into stroke work [akin to kinetic energy] and potential work [which is the energy expended by contraction that does not contribute to stroke volume]. The relationship of stroke work, to total work is represented by mechanical efficiency [figure 2] [6, 7].
Importantly, one variable that determines mechanical efficiency is the elastance [or stiffness] of the artery into which the ventricle ejects. Arterial elastance may be viewed as a ‘lumped’ marker of total arterial impedance – colloquially thought of as ‘afterload [8].’ Abnormally high arterial elastance increases end-systolic volume relative to end-diastolic volume; stroke volume shrinks, mechanical efficiency falls, or less total myocardial work is partitioned into stroke work [figure 2].
Figure 2: Ventriculo-arterial uncoupling. A. demonstrates increased impedance [e.g. ‘afterload’] as arterial elastance [Ea, light blue] rises relative to the end-systolic pressure volume relationship [ESPVR, which also falls here for illustrative purposes]. Note the effect of increased Ea relative to ESPVR, increases end-systolic volume [ESV, compare ESVa to ESVb] which reduces stroke volume and, therefore, stroke work relative to total work [mechanical efficiency]. B. shows normal coupling. Red shade shows ‘potential’ cardiac work [circle 2], blue shade is stroke work [circle 1] and purple shade is cardiac filling work. Note these graphs represent a single cardiac cycle.
Elastic Ergotrauma and Pulmonary Impedance
The link unfolding between the lungs and the heart is that abnormal energy application to the lungs stresses the pulmonary vasculature, raises impedance and uncouples the right ventricle [RV] from the pulmonary artery. While data for these specific effects are lacking, there is good evidence that excessive trans-pulmonary stress afterloads the right ventricle. Much of this data was generated by Francois Jardin and his research group using both the pulmonary artery catheter and echocardiography [1, 9-12]. For example, increasing positive end-expiratory pressure progressively diminishes pulmonary arterial velocity time integral [VTI] and increases right ventricular end-systolic volume [13], trans-pulmonary pressure is directly related to pulmonary outflow impedance and RV end-systolic volume [14], and PEEP titration that increases the driving pressure also dilates the RV and diminishes its output [15]. Further, excessive trans-pulmonary pressure raises RV isovolumic contraction pressure and diminishes its stroke volume [16], which, essentially, defines increased pulmonary arterial elastance and RV-PA uncoupling.
Veno-cardiac Uncoupling
While the maldistribution of myocardial energy, from stroke work to potential work, is typically thought of in terms of the ventricle and arterial system [figure 2], I have argued for the venous corollary [figure 3]. If energy is conserved proportionally, it is expected that energetic uncoupling between the heart and arteries is equally expressed in the veins. As increased arterial elastance diminishes stroke volume for any given cardiac filling pressure, this flattens the Frank-Starling curve on the Guyton analysis. Thus, ventriculo-arterial uncoupling elevates right atrial pressure relative to the mean systemic filling pressure [Pmsf]; cardiac performance [Eh] falls [figure 3] [17, 18]. Analogous to falling stroke work relative to potential work, kinetic venous return work falls relative to potential venous return work. As postulated for the lung, does excessive work over time, directed at the veins, lead to tissue dysfunction?
Figure 3: Veno-cardiac uncoupling on stylized Guyton diagram. A. illustrates effect of increased pulmonary arterial impedance, or elastance. Cardiac performance [Eh] is impaired and the slope flattens, right atrial pressure [Pra] approaches mean systemic filling pressure [Pmsf]. Colour-shading corresponds to figure 2, note how work shifts away from kinetic work [e.g. stroke work] towards potential work. B. illustrates the normal state. Note that if Eh were perfect [1.0] the slope of the Starling curve would be vertical and filling work [purple] would be nil, this is expected if the right heart fills as an unstressed chamber, which it does in health [19]. These graphs are work over time [cardiac output is y-axis] so the shaded areas more specifically reflect power.
Venous Ergotrauma?
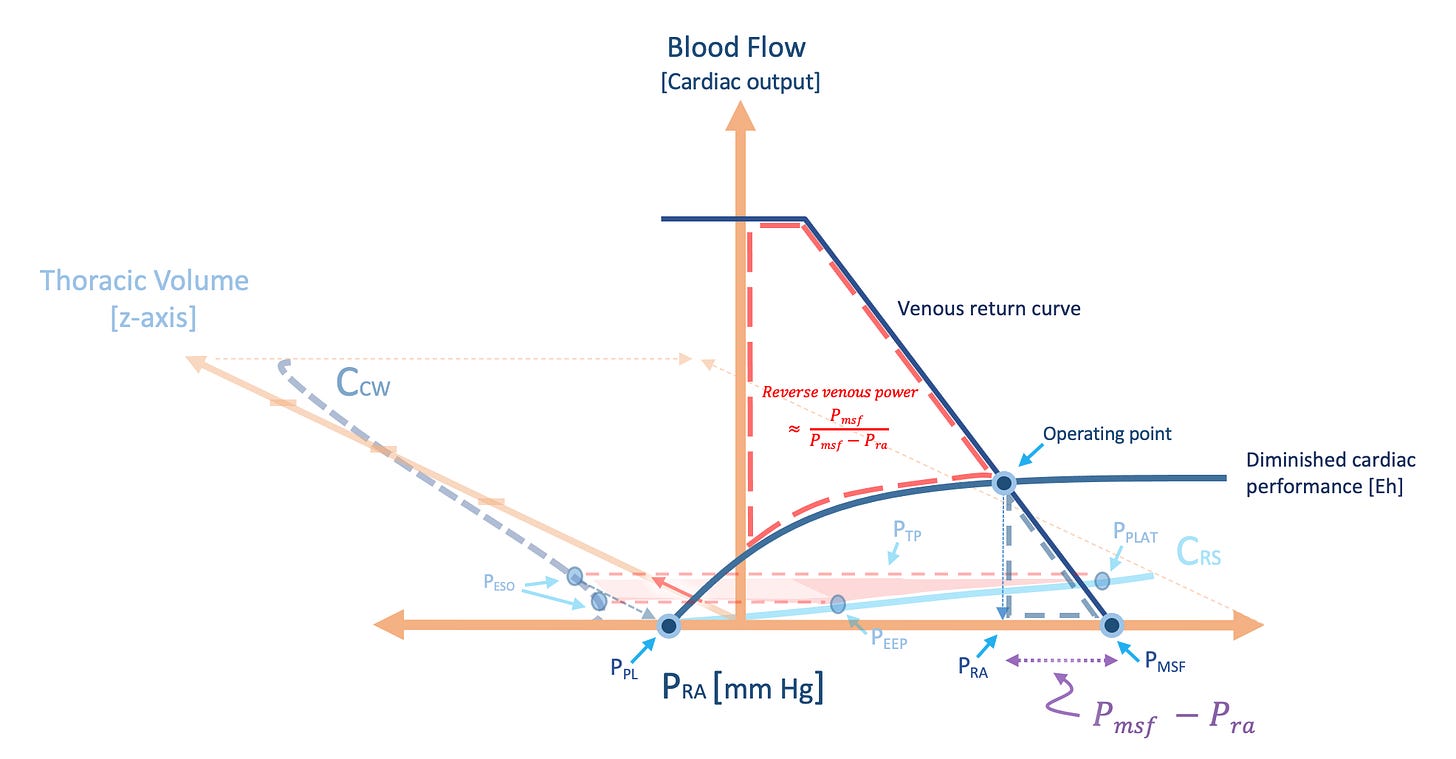
In theory, aberrant potential venous return work acts ‘backwards’ against the venous system; its extent is visualized on a Guyton diagram [figures 3 & 4]. Its value is, therefore, approximately the inverse of cardiac performance [Eh]. Accordingly, ‘reverse’ venous power is directly proportional to Pmsf and inversely proportional to the difference between the Pmsf and right atrial pressure. Inordinate potential venous return work is expected to dilate the great veins to their elastic limit and enhance venous velocity pulsatility – as seen on great vein Doppler [20-23]. Consequently, aberrant venous velocimetry suggests not simply elevated Pmsf [e.g. elevated venous blood volume] but also impaired cardiac performance [i.e. diminished Starling curve slope]. Diuresis may reduce Pmsf and also improve cardiac performance if elevated blood volume also impairs cardiac function [e.g. stretch-induced afterload elevation, functional regurgitation, dysrhythmias]. However, therapy that specifically lowers pulmonary arterial impedance [e.g. optimizing pulmonary driving power, inhaled vasodilators, prone position] or improves cardiac performance, in general [i.e. lower right atrial pressure relative to Pmsf] should also mitigate venous ergotrauma. As always, clinical context steers physiology [figure 4].
Figure 4: Cardiothoracic uncoupling. Figure 1B is now on the x,z plane [into the page] and figure 3A is now on the x,y plane as the Guyton diagram. ‘Alveolo-pleural uncoupling’ is evidenced by excessive area between the Crs and Ccw for the tidal volume [red arrow, z axis] delivered; this afterloads the RV and causes ‘ventriculo-arterial uncoupling’ [VAC]. VAC between the right ventricle and pulmonary artery is illustrated as impaired cardiac performance on the Guyton diagram, or ‘veno-cardiac uncoupling’. Thus, the area bounded by red dashes in the x,y plane represents ‘reverse’ venous power which is approximated by Pmsf and cardiac performance. This power fraction should reveal itself in great vein Doppler velocimetry. See previous figures for abbreviations. The operating point defines the Pra and cardiac output for a given state.
Conclusion
Graphically-linking cardiac and thoracic physiology focuses the interrelations of the heart and the lungs. This mechanical link intimates that energetic uncoupling between the alveolus and pleural space might also uncouple the heart from the vascular system. The thorax is the nexus of mechanical power applied not only to the lung, but also the heart. Accordingly, injurious energetics within the chest reach the tissues via hemodynamics. Both the ‘forward-arterial’ and ‘reverse-venous’ systems are risked by aberrant thoracic power. Future investigation might reveal that efficient energy transfer within the moving organs optimizes these systems; new models are prerequisites for these avenues of inquiry.
Please see other posts in this series and note that my physiology textbook is complete and will be available for free! Its release will be announced on pulmccm.org and on twitter.
JE
Dr. Kenny is the cofounder and Chief Medical Officer of Flosonics Medical; he also the creator and author of a free hemodynamic curriculum at heart-lung.org
References
Jardin F, Vieillard-Baron A: Right ventricular function and positive pressure ventilation in clinical practice: from hemodynamic subsets to respirator settings. In: Applied Physiology in Intensive Care Medicine. Springer; 2006: 207-215.
Cressoni M, Gotti M, Chiurazzi C et al: Mechanical power and development of ventilator-induced lung injury. The Journal of the American Society of Anesthesiologists 2016.
Gattinoni L, Tonetti T, Cressoni M et al: Ventilator-related causes of lung injury: the mechanical power. Intensive care medicine 2016, 42(10):1567-1575.
Marini JJ, Hotchkiss JR, Broccard AF: Bench-to-bedside review: microvascular and airspace linkage in ventilator-induced lung injury. Critical Care 2003, 7(6):435.
Sagawa K: The end-systolic pressure-volume relation of the ventricle: definition, modifications and clinical use. Circulation 1981, 63(6):1223-1227.
Guarracino F, Baldassarri R, Pinsky MR: Ventriculo-arterial decoupling in acutely altered hemodynamic states. Critical Care 2013, 17(2):213.
Guarracino F, Ferro B, Morelli A et al: Ventriculoarterial decoupling in human septic shock. Critical Care 2014, 18(2):R80.
Sunagawa K, Maughan WL, Burkhoff D et al: Left ventricular interaction with arterial load studied in isolated canine ventricle. American Journal of Physiology-Heart and Circulatory Physiology 1983, 245(5):H773-H780.
Jardin F, Vieillard-Baron A: Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive care medicine 2007, 33(3):444-447.
Repessé X, Charron C, Vieillard-Baron A: Acute respiratory distress syndrome: the heart side of the moon. Current opinion in critical care 2016, 22(1):38-44.
Vieillard-Baron A, Matthay M, Teboul J et al: Experts’ opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive care medicine 2016, 42(5):739-749.
Repessé X, Charron C, Vieillard-Baron A: Assessment of the effects of inspiratory load on right ventricular function. Current opinion in critical care 2016, 22(3):254-259.
Jardin F, Brun-Ney D, Hardy A et al: Combined thermodilution and two-dimensional echocardiographic evaluation of right ventricular function during respiratory support with PEEP. Chest 1991, 99(1):162-168.
Vieillard-Baron A, Loubieres Y, Schmitt JM et al: Cyclic changes in right ventricular output impedance during mechanical ventilation. J Appl Physiol (1985) 1999, 87(5):1644-1650.
Schmitt J-M, Vieillard-Baron A, Augarde R et al: Positive end-expiratory pressure titration in acute respiratory distress syndrome patients: impact on right ventricular outflow impedance evaluated by pulmonary artery Doppler flow velocity measurements. Critical care medicine 2001, 29(6):1154-1158.
Jardin F, Brun-Ney D, Cazaux P et al: Relation between transpulmonary pressure and right ventricular isovolumetric pressure change during respiratory support. Catheterization and cardiovascular diagnosis 1989, 16(4):215-220.
Parkin WG, Leaning MS: Therapeutic control of the circulation. Journal of clinical monitoring and computing 2008, 22(6):391-400.
Walley KR: Left ventricular function: time-varying elastance and left ventricular aortic coupling. Critical care 2016, 20(1):270.
Pinsky MR: My paper 20 years later: Effect of positive end-expiratory pressure on right ventricular function in humans. Intensive care medicine 2014, 40(7):935-941.
Beaubien‐Souligny W, Benkreira A, Robillard P et al: Alterations in Portal Vein Flow and Intrarenal Venous Flow Are Associated With Acute Kidney Injury After Cardiac Surgery: A Prospective Observational Cohort Study. Journal of the American Heart Association 2018, 7(19):e009961.
Denault AY, Beaubien-Souligny W, Elmi-Sarabi M et al: Clinical Significance of Portal Hypertension Diagnosed With Bedside Ultrasound After Cardiac Surgery. Anesthesia & Analgesia 2017, 124(4):1109-1115.
Beaubien-Souligny W, Denault A, Robillard P et al: The Role of Point-of-Care Ultrasound Monitoring in Cardiac Surgical Patients With Acute Kidney Injury. Journal of Cardiothoracic and Vascular Anesthesia 2018.
Beaubien-Souligny W, Rola P, Haycock K et al: Quantifying systemic congestion with Point-Of-Care ultrasound: development of the venous excess ultrasound grading system. The ultrasound journal 2020, 12:1-12.